Volume 19, Number 5—May 2013
Dispatch
Contaminated Ventilator Air Flow Sensor Linked to Bacillus cereus Colonization of Newborns
Cite This Article
Citation for Media
Abstract
We investigated Bacillus cereus–positive tracheal aspirates from infants on ventilators in a neonatal intensive care unit. Multilocus sequence typing determined a genetic match between strains isolated from samples from a case-patient and from the air flow sensor in the ventilator. Changing the sterilization method for sensors to steam autoclaving stopped transmission.
Because of ubiquity in the environment, the recovery of Bacillus species from clinical specimens is often considered a clinically inconsequential contamination. Nevertheless, an accumulating body of literature suggests that contamination with this organism should not be routinely dismissed (1). Severe and lethal Bacillus cereus infections have been described in newborn infants, with higher frequency among premature infants. The types of B. cereus infections in newborns included central nervous system, respiratory tract, primary bacteremia, and sepsis (2–4). Nosocomial outbreaks of B. cereus implicating hospital linens, manual ventilation balloons, contaminated diapers, and contaminated ventilator equipment have also been reported (5–9).
The Missouri Department of Health and Senior Services conducted this investigation in response to the hospital’s identification of an increased number of tracheal aspirates that were positive for B. cereus collected from newborns who were on ventilators during March–May, 2011. All tracheal aspirate culture results obtained in the Neonatal Intensive Care Unit (NICU) during January 2010–June 2011 were reviewed. NICU data was also searched for positive B. cereus culture from other specimens, such as blood, body fluids, or tissues. Investigators thoroughly evaluated respiratory management practices in the unit by direct observation, respiratory records review, and an interview with the respiratory therapist.
Several environmental cultures were obtained from the flow sensors of the unit’s ventilators over the 1-month period. B. cereus isolates were forwarded to the Centers for Disease Control and Prevention to be molecularly characterized by using multilocus sequence typing (MLST) (10). DNA was prepared from bacterial cultures as described (11). The DNA was used as a template in PCRs with the primers described on the Bacillus cereus MLST Web site (www.pubmlst.org/bcereus) for the 7 loci which define the MLST scheme. The sequences for the loci glpF, gmk, ilvD, pta, pur, pycA, and tpi were then assigned allele designations. The combination of the 7 alleles determines a given sequence type. A greater number of alleles that match between strains indicates a higher level of relatedness (10). Prevalence of B. cereus–positive specimens was compared by using the Mann-Whitney U test.
Retrospective analysis of tracheal aspirate culture results showed significant increase (p = 0.039) in B. cereus isolation between March and May, 2011 (Figure 1). No Bacillus spp. were isolated from blood, other body fluids, or tissues during the study period. The chart review of the case-patients comprising the cluster of B. cereus colonization revealed that none received a diagnosis of clinical B. cereus infection. All patients were treated with vancomycin or tobramycin, or both, for indications not related to B. cereus in tracheal aspirate. One case-patient died 108 days later without evidence that B. cereus contributed to the outcome. All other case-patients recovered and were discharged.
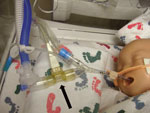
Investigation of the ventilation procedures in the NICU revealed that most equipment used for respiratory care was disposable, designated for single-patient use. The Draeger Evita v500 ventilator (Draeger Medical Inc., Telford, PA, USA; www.draeger.us/sites/enus_us/pages/hospital/evita-xl.aspx) was used for mechanical ventilation of infants who were intubated to treat severe respiratory compromise. The Draeger Evita V500 is a microprocessor controlled ventilator offering both mandatory and spontaneous ventilation modes for adult, pediatric, and neonatal patients. Heated and humidified gas flows from the ventilator unit, through the inspiratory circuit and NeoFlow air flow sensor to the patient through an endotracheal tube. Upon exhalation, gas flows back through the air flow sensor into the expiratory circuit and returns to the ventilator through the expiratory flow sensor and exhalation valve. In addition to the ventilator, reusable respiratory equipment comprised a proximal air flow sensor, expiratory flow sensor, exhalation valve, and circuit temperature probe. The sensor closest to the newborn’s mouth was an air flow sensor located inside the disposable ventilation circuit (Figure 2). From 9 environmental cultures obtained from 9 air flow sensors, 1 was positive for Bacillus spp., and was later confirmed as B. cereus by the State Public Health Laboratory.
MLST was performed for 8 B. cereus isolates from case-patients and for 1 environmental isolate from the air flow sensor. We were able to fully characterize 4 of the 9 isolates (Table). One locus for the remaining 5 strains did not yield an amplicon for sequencing after repeated attempts and, thus, could not be assigned a sequence type. The isolates that included sequence type (ST) 73 and ST94 were closely related to each other because they differed by merely 1 locus, gmk. The strains that were not fully typed because of the inability to obtain sequences for locus pta were also closely related to ST73 or ST94 because the other loci matched. There was 1 match between strains isolated from 1 case-patient and the air flow sensor, which was ST73. The contaminated air flow sensor was then sterilized by using a steam autoclave. A repeat culture of this sensor after sterilization was negative.
We found that air flow sensors were routinely disinfected by placing them in a container with 70% alcohol solution for 60 minutes. After discovery of the air flow sensor contaminated with B. cereus, the disinfection policy was changed. All air flow sensors were first soaked in Enzol enzymatic detergent (ASP, Irvine, CA, USA; www.aspjj.com/us/products/enzol) solution and then sent for steam autoclave sterilization at 134°C (273.2°F). After implementation of new disinfection and sterilization procedures, no new cases of B. cereus tracheal colonization were identified in the nursery. In this cluster, contaminated proximal air flow sensors were the likely source of tracheal colonization with B. cereus in newborn infants, supported by a genetic match by MLST between a strain isolated from 1 case-patient and the contaminated air flow sensor.
B. cereus transmission from contaminated respiratory equipment has been reported in other geographic areas. In the Netherlands, an outbreak of B. cereus infections in a pediatric intensive care unit caused by contaminated reusable ventilator air flow sensors was described (7). Switching to disposable air flow sensors stopped colonization with B. cereus in that unit. In Canada, an outbreak of B. cereus infections among patients in an adult ICU was linked to colonized ventilator circuitry (8). In the United Kingdom, reusable ventilator circuits were also identified as the cause of a B. cereus outbreak among intubated NICU patients (9).
Our investigation underscores the necessity of close monitoring of occurrences of Bacillus spp. in tracheal aspirates since clustering of such cases could be an indication of single source contamination. In our investigation, B. cereus isolates were either ST73, ST94, or closely related to those sequence types. ST73 and ST94 are associated with strains previously described as having caused illness in elderly persons. Strains with ST73 were implicated in cases of septicemia (12), and of sepsis and pneumonia (13). Strains with ST 94 were recovered from patients with pneumonia (14). B. cereus strains harboring B. anthracis plasmids such as pXO1, have also been associated with severe and fatal respiratory infections (15).
All case-patients in our investigation were considered to be colonized with B. cereus without clinical implications. Since all of them received intravenous antimicrobial drugs effective against B. cereus, it is conceivable that the clinical course of those patients could have been different without such treatment.
Bacillus spp. in tracheal aspirate cultures should not be routinely viewed as clinically insignificant and further testing to determine exact strain should be considered under appropriate clinical and epidemiologic circumstances. Proper disinfection of the entire ventilator circuit as recommended by the equipment manufacturer is crucial in avoiding potentially lethal B. cereus infections.
Dr Turabelidze is a Missouri State Epidemiologist. His main research interests include epidemiology of communicable diseases.
Acknowledgment
This study made use of the Bacillus cereus Multi Locus Sequence Typing website (http://pubmlst.org/bcereus/) developed by Keith Jolley and sited at the University of Oxford. The development of that site was funded by the Wellcome Trust.
References
- Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382–98. DOIPubMedGoogle Scholar
- Manickam N, Knorr A, Muldrew KL. Neonatal meningoencephalitis caused by Bacillus cereus. Pediatr Infect Dis J. 2008;27:843–6. DOIPubMedGoogle Scholar
- Lebessi E, Dellagrammaticas HD, Antonaki G, Foustoukou M, Iacovidou N. Bacillus cereus meningitis in a term neonate. J Matern Fetal Neonatal Med. 2009;22:458–61. DOIPubMedGoogle Scholar
- Hilliard NJ, Schelonka RL, Waites KB. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol. 2003;41:3441–4. DOIPubMedGoogle Scholar
- Sasahara T, Hayashi S, Morisawa Y, Sakihama T, Yoshimara A, Hirai Y. Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis. 2011;30:219–26. DOIPubMedGoogle Scholar
- Van Der Zwet WC, Parlevliet GA, Savelkoul PH, Stoof J, Kaiser AM, van Furth AM, Outbreak of Bacillus cereus infections in a neonatal intensive care unit traced to balloons used in manual ventilation. J Clin Microbiol. 2000;38:4131–6 .PubMedGoogle Scholar
- Kalpoe JS, Hogenbrick K, van Maarseveen NM, Gesink-Van der Veer BJ, Kraakman MEM, Maarleveld JJ, Dissemination of Bacillus cereus in a pediatric intensive care unit traced to insufficient disinfection of reusable ventilator air-flow sensors. J Hosp Infect. 2008;68:341–7. DOIPubMedGoogle Scholar
- Bryce EA, Smith JA, Tweeddale M, Andruschak BJ, Maxwell MR. Dissemination of Bacillus cereus in an intensive care unit. Infect Control Hosp Epidemiol. 1993;14:459–62. DOIPubMedGoogle Scholar
- Gray J, George RH, Durbin GM, Ewer AK, Hocking MD, Morgan ME. An outbreak of Bacillus cereus respiratory tract infections on a neonatal unit due to contaminated ventilator circuits. J Hosp Infect. 1999;41:19–22. DOIPubMedGoogle Scholar
- Priest FG, Barker M, Baillie LW, Holmes EC, Maiden MC. Population structure and evolution of the Bacillus cereus group. J Bacteriol. 2004;186:7959–70. DOIPubMedGoogle Scholar
- Hoffmaster AR, Fitzgerald CC, Ribot E, Mayer LW, Popovic T. Molecular subtyping of Bacillus anthracis and the 2001 bioterrorism-associated anthrax outbreak, United States. Emerg Infect Dis. 2002;8:1111–6. DOIPubMedGoogle Scholar
- Barker M, Thakker B, Priest FG. Multilocus sequence typing reveals that Bacillus cereus strains isolated from clinical infections have distinct phylogenetic origins. FEMS Microbiol Lett. 2005;245:179–84. DOIPubMedGoogle Scholar
- Vassileva M, Torii K, Oshimoto M, Okamoto A, Agata N, Yamada K, Phylogenetic analysis of Bacillus cereus isolates from severe systemic infections using multilocus sequence typing scheme. Microbiol Immunol. 2006;50:743–9 .PubMedGoogle Scholar
- Hoffmaster AR, Novak RT, Marston CK, Gee JE, Helsel L, Pruckler JM, Genetic diversity of clinical isolates of Bacillus cereus using multilocus sequence typing. BMC Microbiol. 2008;8:191. DOIPubMedGoogle Scholar
- Hoffmaster AR, Hill KK, Gee JE, Marston CK, De BK, Popovic T, Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J Clin Microbiol. 2006;44:3352–60. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 19, Number 5—May 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
George Turabelidze, Missouri Department of Health and Senior Services, Eastern District Office, 220 S Jefferson St, St Louis, MO, 63103, USA
Top