Volume 2, Number 1—January 1996
Synopsis
Emergence of the Ehrlichioses as Human Health Problems
Abstract
Ehrlichiae are small, gram-negative, obligately intracellular bacteria that reside within a phagosome. The first human ehrlichial infection was recognized in the United States in 1987. It was later shown to be caused by a new species, Ehrlichia chaffeensis. In 1994, an ehrlichial pathogen within neutrophils that is closely related to the known veterinary pathogens E. equi and E. phagocytophila was found to infect humans. Molecular methods were required to detect, characterize, and identify these fastidious and uncultivated bacteria. Subsequently, E. chaffeensis infection was documented in more than 400 patients in 30 states, Europe, and Africa. Likewise, approximately 170 cases of human granulocytic ehrlichiosis have been diagnosed, most since 1994, predominantly in the upper midwestern and northeastern states, but also in northern California. The disease caused by ehrlichiae is generally undifferentiated but is often associated with leukopenia, thrombocytopenia, and elevated serum hepatic transaminase levels in tick-exposed patients. Infection ranges from subclinical to fatal; tetracycline appears to be an effective therapy. The emergence of these two newly recognized tickborne infections as threats to human health is probably due to increased clinical cognizance, but as in other emerging tickborne infections, it is likely that the rapid increase in identified cases signals a true emergence of disease associated with a changing vector-host ecology.
During the last decade, two previously unknown human diseases caused by Ehrlichia species have emerged as public health problems in the United States. Each of these infectious diseases is designated by the major target cell: human monocytic ehrlichiosis is caused by Ehrlichia chaffeensis, and human granulocytic ehrlichiosis by an E. equi-like organism. The taxonomic positions of these bacteria have been determined with much greater scientific clarity by the recent application of molecular methods, which were critical for the identification of E. chaffeensis and essential for that of human granulocytic ehrlichia (HGE) (1,2). Extreme difficulty in developing methods for cultivation of these ehrlichiae and, until recently, the lack of molecular approaches to the study of uncultivated organisms partly explain why these far from rare diseases were not detected sooner (3-5). However, it was the observation of Romanowsky-stained blood smears by classic microscopy that actually opened the door to the discovery of both diseases (6,7). This article reviews ehrlichiae and describes their taxonomic and ecologic niches within the scheme of bacterial evolution and the substantial knowledge, developed largely by the veterinary sciences, that served as a foundation for the discovery and elucidation of the human ehrlichioses.
Among the obligately intracellular gram-negative bacteria, a genetically related set is classified among the Protobacteria of the subgroup on the basis of sequence analysis of the 16S rRNA gene (1,2,8,9). The overview of the evolution of these organisms provided by this approach lacks many details essential to the understanding of human disease; however, the evolution of these organisms correlates well with the clonal divergence of many species that do not have opportunities for genetic recombination because of their intracellular isolation from other organisms. These bacteria have evolved in close association with ticks, mites, chiggers, fleas, other arthropods, and fish flukes into six genetically defined clusters (1,2,8-11) (Table 1 and Figure 1). Future definition of the details determined by many other important genes may designate these six clusters as separate taxonomic genera. Currently, however, the presence of organisms named as Ehrlichia species within three of these clusters is confusing, particularly since other genus names also fall into each of the clusters (Table 1 and Table 2).
Historical Foundations for Recognizing the Human Ehrlichioses in the United States
The current, albeit incomplete, knowledge of the human ehrlichioses has a long history. The emergence of ehrlichiae began in 1910, when Theiler described Anaplasma marginale, the etiologic agent of an economically important, severe worldwide disease of cattle. The contributions of veterinary medical science to the understanding of ehrlichiae have continued ever since; they include the description of Cowdria ruminantium by Cowdry in 1925, of E. canis by Donatien and Lestoquard in 1935, and E. phagocytophila by Gordon in 1940. The genus Ehrlichia was established in 1945 in honor of the German microbiologist Paul Ehrlich, and the general scientific distinction of rickettsiae and ehrlichiae from viruses and protozoa followed soon thereafter, mainly as a result of the advent of antibiotics and electron microscopy. Molecular methods are now used to identify various species. Indeed, E. sennetsu, the etiologic agent of a human infectious mononucleosis-like illness described in Japan in 1954, was considered a Rickettsia for many years. Numerous veterinary discoveries preceded the recognition of human ehrlichial infections in the United States: Neorickettsia helminthoeca in 1950, N. elokominica in 1964, E. equi in 1969, E. ewingii in 1971, E. platys in 1978, and E. risticii in 1984 (12). Substantial emphasis on the study of ehrlichiae was stimulated by a disastrous epizootic of canine ehrlichiosis that resulted in 200 to 300 deaths among military working dogs in Vietnam from 1968 until 1970. After the discovery, in 1984, that the etiologic agent of Potomac horse fever was a novel species designated as E. risticii, major advances in the scientific investigation of ehrlichiae have been achieved, particularly in the laboratory of Yasuko Rikihisa at the Ohio State University College of Veterinary Medicine. Indeed, blinded to the potential relevance of Ehrlichia ssp. to human medicine, we missed numerous opportunities during the 1980s to investigate canine ehrlichiosis.
Emergence of Human Monocytic Ehrlichiosis
A single, somewhat unusual case in 1986 led to the recognition of human ehrlichial infections in the United States (6). A 51-year-old man who had been bitten by a tick in Arkansas and had been sick for 5 days was admitted to a hospital in Detroit. He was critically ill with fever, headache, myalgia, confusion, azotemia, hypoxemia, thrombocytopenia and had cytoplasmic inclusions in his peripheral blood leukocytes. Peripheral blood smears and electron micrographs of the inclusion-bearing leukocytes examined at the Centers for Disease Control (CDC) suggested that the organisms were an Ehrlichia sp. Serologic evaluation showed a high titer of antibodies reactive with E. canis that fell sharply during convalescence. The patient had a prolonged hospitalization complicated by renal failure which required hemodialysis; upper gastrointestinal hemorrhage; severe central nervous system involvement; and systemic Candida infection.
State public health agencies and CDC demonstrated that many patients originally suspected to have Rocky Mountain spotted fever or another rickettsiosis (13) produced antibody to E. canis, a canine pathogen. In Oklahoma, the state with the highest incidence of Rocky Mountain spotted fever, serologic evidence suggested that human monocytic ehrlichiosis occurred at the same incidence as Rocky Mountain spotted fever (14). In a prospective active surveillance study of febrile hospitalized patients in southeastern Georgia, ehrlichial disease was sixfold more prevalent than Rocky Mountain spotted fever and was associated with history of tick bite, anorexia, chills, weight loss, sweating, headache, nausea, myalgia, thrombocytopenia, and elevated serum concentrations of hepatic transaminases in 50% or more of the patients and by arthralgia, vomiting, diarrhea, abdominal pain, cough, rash, and leukopenia in fewer than half of the patients (15). Mild and asymptomatic seroconversion to ehrlichial antigens has also been associated with tick exposure (16).
A practical method for the cultivation of E. canis (the suspected cause of human ehrlichial infections), developed at CDC, used the cultivated organisms to establish a reliable indirect immunofluorescence assay (IFA) for the diagnostic demonstration of antibodies in serum (17). In retrospect, it is apparent that E. canis was a relatively sensitive surrogate antigen because it shares some, but by no means all, of the antigens of the etiologic Ehrlichia. The method of ehrlichial cultivation in a continuous canine histiocytoma cell line (DH82) was also successfully applied to the isolation of an Ehrlichia species from the blood of a febrile soldier at Fort Chaffee, Arkansas (3). Molecular methods were also applied to establish, on the basis of the DNA sequence of the 16S rRNA gene, that E. chaffeensis is a novel species (1).
Microbiology of the Etiologic Agent
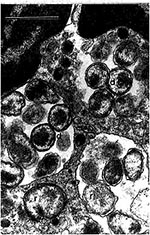
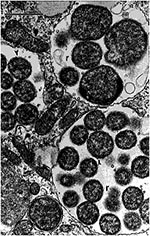
Ultrastructural investigation of E. chaffeensis demonstrated that the inner and outer leaflets of the cell wall were equal in thickness. Rickettsia species have a thinner outer leaflet and thicker inner leaflet, and Orientia (Rickettsia) tsutsugamushi has a thicker outer leaflet and thinner inner leaflet (18-20) (Figure 2 and Figure 3). Two distinct morphologic forms, larger reticulate cells with uniformly dispersed nucleoid filaments and ribosomes and smaller cells with central condensation of nucleoid filaments and ribosomes, were observed undergoing binary fission, evidence against a developmental cycle (Figure 3). Many forms were observed, including numerous intramorular vesicles and tubules originating from the ehrlichial cell wall and fibrillar material consisting of ehrlichial antigen. Abnormal forms included giant and multilobar ehrlichiae.
Western immunoblot technique has detected seven major proteins: 120, 66, 58, 44, 29, 28, and 22 kDa; the greatest number of antigens are shared with E. canis (21). Monoclonal antibodies and monospecific polyclonal antibodies demonstrated that the major, immunodominant 120-, 29-, 28-, and 22-kDa proteins (as well as a minor 30-kDa protein) are surface-exposed and that the 28- and 22-kDa proteins are related antigenically. Further human isolations of ehrlichiae have shown antigenic and genetic diversity among strains of E. chaffeensis (4).
DNA cloning has shown that 58- and 10-kDa proteins are genetically homologous to the Escherichia coli GroEL and GroES heat shock proteins (22). The 120-kDa protein includes a region of identical 80 amino acid tandem repeat units, and preliminary evidence suggests that it mediates adhesion to the host cell. Establishment and study of tick isolates and identification of virulence factors are clearly important aims for future research.
Clinical Manifestations
Most cases of human ehrlichiosis have been diagnosed after a moderate-to-severe illness (23). Some patients have a life-threatening illness resembling toxic shock syndrome (24). Deaths have occurred in approximately 2% to 3% of patients, including previously healthy children (13,23-28). Among 237 cases between 1985 and 1990 investigated by CDC, 62% of patients were hospitalized (23). The median duration of illness, including that for treated patients, was 23 days. The signs and symptoms depict a systemic disease that has no clinically diagnostic features: fever (97%), headache (81%), myalgia (68%), anorexia (66%), nausea (48%), vomiting (37%), rash (6% at onset, 25% during the first week, and 36% overall), cough (26%), pharyngitis (26%), diarrhea (25%), lymphadenopathy (25%), abdominal pain (22%), and confusion (20%). Severe complications include respiratory and renal insufficiency and serious neurologic involvement. Of patients with chest radiographic examinations, nearly half have pulmonary infiltrates (13). Clinical laboratory findings include leukopenia (60%), thrombocytopenia (68%), and elevated hepatic transaminases (86%). Hepatic involvement has been described as severe in individual cases (29). Central nervous system involvement has been documented by the occurrence of seizures, coma, and cerebral lesions at autopsy as well as by cerebrospinal fluid (CSF) pleocytosis, increased CSF protein concentration, and the presence of E. chaffeensis in CSF demonstrated by immunocytology and polymerase chain reaction (PCR) (24,30,31). Recognition of serious myocardial involvement further emphasizes the potential gravity of this disease and the incompleteness of our knowledge of its clinical manifestations (32). Seroconversion to E. chaffeensis among 1.3% of 1,187 soldiers during training exercises with tick exposures suggests that human risk for infection with it or an antigenically related Ehrlichia is relatively high (16). However, the fact that two-thirds of the seroconverters remained asymptomatic emphasizes that the relationship between severity of illness and either host factors or ehrlichial strain differences in virulence remains unknown.
Epidemiology of Human Monocytic Ehrlichiosis and Ecology of E. chaffeensis
More than 400 cases of serologically confirmed E. chaffeensis infection have been documented at CDC, which has one of the few laboratories capable of performing diagnostic ehrlichial serology. That these cases most likely represent but the tip of the iceberg was confirmed when inquiry at MRL Diagnostics, a commercial reference laboratory that offers IFA serology for E. chaffeensis, reported 722 positive specimens between September 1992 and June 15, 1995. In fact, there is no system, required or otherwise, for notifying public health authorities of cases. Relatively few physicians even know that these diseases exist and, thus, most of them are not making the diagnosis. Even highly knowledgeable physicians find ehrlichiosis virtually impossible to diagnose on the basis of clinical signs and symptoms. Most patients (83%) report exposure to ticks or tick bite within the 3 weeks of onset of illness (13,23). Cases are predominantly rural (66%) and seasonal (68% during May-July). The median age of patients is 44 years, and three-quarters are male. Outbreaks of human monocytic ehrlichiosis among groups of golfers and campers emphasize the risk for infection during outdoor activities with tick exposure (16,33). Human monocytic ehrlichiosis occurs not only as an acute illness of apparently immunocompetent persons but also as an opportunistic infection of patients with compromised host defenses, including acquired immunodeficiency syndrome (AIDS) patients (24,26). The report of a fatal E. chaffeensis infection in an AIDS patient, in whom a diagnostic antibody response to E. chaffeensis never developed, suggests that such cases would not usually be diagnosed correctly.
E. chaffeensis has been detected in two tick species, Amblyomma americanum (the lone star tick) and Dermacentor variabilis (34,35) (the American dog tick). Human monocytic ehrlichiosis has been confirmed in 30 states. Most cases have occurred within the range of A. americanum, and a high proportion of the remainder have occurred within the range of D. variabilis. Ehrlichiae have been detected specifically by PCR in adult (but not in nymphs) A. americanum ticks in Missouri, North Carolina, Kentucky, and New Jersey; a single E. chaffeensis PCR-positive D. variabilis tick was found in Arkansas. On the other hand, cases reported in Wyoming, Utah, Washington, Europe, and Africa suggest the possibility of additional vectors (23,36,37) or other antigenically related ehrlichial organisms.
White-tailed deer (Odocoileus virginianus) in Alabama, Arkansas, Florida, Georgia, Illinois, Kentucky, Louisiana, Maryland, Mississippi, Missouri, North Carolina, South Carolina, Tennessee, Texas, and Virginia have antibodies reactive with E. chaffeensis. These deer are susceptible to experimental infection with ehrlichiae that can circulate for weeks (38,39). The close association among E. chaffeensis, the lone star tick, and white-tailed deer was vividly illustrated by the sequential appearance of A. americanum ticks and antibodies to E. chaffeensis in a population of deer in Georgia (40). The prevalence of lone star ticks and seropositivity both rose from 1983 to 1990 when 100% of deer examined were infested with A. americanum and had serum antibodies to E. chaffeensis. The possibility that the immature ticks acquired the ehrlichiae from another mammalian host, such as a small rodent, before it was transmitted to deer cannot be excluded. Experimentally infected dogs have ehrlichemia for at least 26 days and, thus, could also serve as a reservoir host (41). Related ehrlichioses for which the ecology and transmission are known are maintained in a cycle involving a mammalian host and a tick vector (12). For example, Rhipicephalus sanguineus ticks acquire E. canis infection when feeding on infected dogs as larvae or nymphs (42,43). Although the ticks remain infected as they molt from stage to stage, transovarian transmission does not occur. It seems likely that deer, dogs, or small rodents serve as the reservoir hosts for E. chaffeensis, and that A.americanum ticks serve as the major vector. Indeed, larval and nymphal A. americanum acquire E. chaffeensis by feeding on infected deer, maintain the ehrlichiae transstadially, and transmit E. chaffeensis while feeding as nymphs and adults on naive deer (44).
Diagnosis and Treatment
The diagnosis of human monocytic ehrlichiosis is usually difficult to establish during the acute stage of the infection, even in severe cases. Ehrlichiosis should be considered in any febrile patient who has been exposed to ticks during the previous 3 weeks, particularly if leukopenia and thrombocytopenia are present.
Most cases have been confirmed by a humoral immune response generating antibodies reactive with E. chaffeensis or the surrogate antigen, E. canis (17,23). Use of E. chaffeensis antigens for IFA serology results in greater diagnostic sensitivity than use of E. canis antigens (3). Diagnostic rises in antibody titer usually occur by the third week after onset, and a precipitous decline in antibody occurs in most patients during the following year. A fourfold rise or fall in IFA titer with a peak titer of 64 or greater is considered diagnostic in a clinically compatible case. Only two cases have been documented by ehrlichial isolation (3,4), approximately 30 cases by E. chaffeensis-specific PCR (31,34,45), and even fewer cases by immunohistology or immunocytology (25-28,30,46). Furthermore, antibodies to E. chaffeensis did not develop in six PCR-confirmed patients during convalescence, suggesting that serologic testing may be less sensitive than generally assumed (31,45). The sensitivity of PCR seems to be 80% to 87%; the specificity depends critically upon avoiding contamination with ehrlichial DNA. Search for morulae of E. chaffeensis in leukocytes is unrewarding. Even an exhaustive search of buffy coat smears seldom yields a diagnostic result.
Retrospective analysis showed that treatment with tetracycline was associated with reduced need for hospitalization of patients and with the shortest median duration of treatment to effect defervescence for hospitalized patients (2 days as compared with 3 days for chloramphenicol and 7 days for all other antibiotics) (23). E. chaffeensis is killed in cell culture in the presence of doxycycline or rifampin but is resistant to chloramphenicol, ciprofloxacin, erythromycin, cotrimoxazole gentamicin, and penicillin (47). E. chaffeensis can establish persistent infection even after treatment with tetracycline and chloramphenicol (25). Persistent ehrlichial infection, in some instances even after treatment, is a well-documented aspect of the veterinary ehrlichioses, e.g., canine monocytic ehrlichiosis (E. canis) and tick-borne fever (E. phagocytophila). The long-term implications of persistent ehrlichiosis in humans are unclear but might include subsequent reactivation of infection or altered host defenses.
Pathology, Pathogenesis, and Immunity
E. chaffeensis is introduced into the dermis by the bite of an infected tick and spreads hematogenously throughout the body. Intracellular infection is established within phagosomes, most often in macrophages in the spleen, liver, lymph nodes, bone marrow, lung, kidney, and cerebrospinal fluid (25-28,30,46). Lesions potentially attributable to ehrlichial infection include focal necroses of the liver, spleen, and lymph nodes; multiorgan perivascular lymphohistocytic infiltrates; hemophagocytosis in the spleen, liver, lymph nodes, and bone marrow; interstitial pneumonitis; and pulmonary hemorrhage. In bone marrow specimens from 12 patients, the most important findings related to the hematopoietic response were myeloid hyperplasia (8 cases), myeloid hypoplasia (1 case), pancellular hypoplasia (1 case), and megakaryocytosis (7 cases) (46). The most striking discovery was the frequent occurrence of granulomas (in 8 cases) and marrow histiocytosis (in 1 case) as manifestations of the reaction of macrophages to this organism.
The pathogenic mechanisms of ehrlichial disease are poorly understood. E. chaffeensis directly causes necrosis of heavily infected cells in vitro and in immunocompromised patients (7,26); however; the role of host immune and inflammatory responses as disease mechanisms has yet to be determined. Observations of opportunistic fungal and viral infections in severe and fatal cases suggest the possibility of an ehrlichial role in the suppression or dysregulation of the immune response (6,25).
It is quite likely that E. chaffeensis is controlled by a combination of cell-mediated and humoral immune mechanisms. Interferon-activated human monocytes kill E. chaffeensis in vitro. The ehrlichicidal activity is reversed by holotransferrin, suggesting that E. chaffeensis is inhibited by intracellular iron depletion (48). The closely related E. canis organisms, which grow in canine macrophages in the presence of normal canine serum and cause macrophage necrosis, are killed by canine macrophages when the ehrlichiae are opsonized by immune serum (49,50). Among patients who are treated successfully with an anti-ehrlichial drug, the disease-associated lymphocytopenia is corrected and within 2 to 3 days lymphocytosis develops (51). The predominant (range, 41% to 97%) lymphocyte population comprises T lymphocytes, cells that usually constitute only 3% to 8% of peripheral lymphocytes at the institution where the patients were studied. The function of these cells in this setting is unclear as are the consequences of the low T-lymphocyte concentration.
Human granulocytic ehrlichiosis was recognized originally in Duluth, Minnesota, by Johan Bakken as a clinical syndrome of a potentially fatal febrile illness in which the patient's neutrophils contained cytoplasmic inclusions (7). Upon reading an update on human ehrlichioses at the time of the index case, which had a dramatic fatal course, Bakken hypothesized that the infection might have been ehrlichiosis. Collaboration with the authors, who were working together at the University of Texas Medical Branch at Galveston, resulted in the evaluation of a series of patients who were suspected by Bakken to have the disease. Cytoplasmic inclusions were observed in neutrophils, which differed from the findings in E. chaffeensis infections, in which the monocyte/macrophage is the principal target cell and the detection of circulating leukocytes with inclusions is a rare event. Moreover, IFA tests for antibodies to E. chaffeensis performed in the Texas laboratory and subsequently in Dumler's laboratory at the University of Maryland were uniformly negative (4).
In retrospect, June 18, 1992, was a significant day in the history of human granulocytic ehrlichiosis. It was Dumler's last day as a fellow in Galveston before moving to Baltimore to become an assistant professor and establish his own independent ehrlichial research laboratory, and it was Sheng-min Chen's first day as a fellow. Momentously, the blood of a 78-year-old Wisconsin man was collected and sent to Galveston by Bakken on the same day. Culture for ehrlichiae and acute- and convalescent-phase serologic assays for chaffeensis, E. canis, E. sennetsu, and E. risticii were all negative. PCR amplification of the 16S rDNA, the approach developed by Wilson (52) and applied by Relman and co-workers to identify the etiologic agent of bacillary angiomatosis (53), was performed successfully on the specimen by Chen 3 months later (2). Sequencing the gene was not a high priority compared with pursuit of the research aims of funded projects during the season when the ticks were expected to be less active. By April of 1993, DNA sequencing of half the 16S rDNA had been accomplished, and it was recognized that the organism was most closely related to E. phagocytophila and E. equi, closely related to E. platys, less closely related to E. chaffeensis, and distantly related to E. sennetsu. Completion and repeated confirmation of sequencing of both sense and antisense strands enabled genogroup-specific primers to be designed for nested PCR in July 1993.
What had progressed as a collaborative project became the major project in Dumler's laboratory as Chen and Walker refocused their efforts on funded E. chaffeensis research. The initial joint publications with Bakken documented 12 cases diagnosed by specific PCR and the identification of morulae in circulating neutrophils (7). Two patients had died. Necropsy performed on one patient showed ultrastructurally and immunohistologically identified ehrlichiae in neutrophil phagosomes in the spleen. Convalescent-phase IFA serology with surrogate antigens harvested from the blood of an E. equi-infected horse and E. phagocytophila-infected sheep demonstrated antibodies in 9 of the 10 survivors (7). The illness was characterized by chills, fever, myalgias, and headache; some patients also had nausea, confusion, cough, and arthralgias. Laboratory data included leukopenia in 50%, neutropenia in 17%, lymphopenia in 17%, anemia in 50%, thrombocytopenia in 92%, and elevated aspartate aminotransferase in 91%. Patients were predominantly older men (mean age, 68 years). Clinical history showed strong association with tick bite preceding the onset of illness.
Microbiology of Human Granulocytic Ehrlichia and E. equi
Evidence is accumulating to support the premise that a single Ehrlichia species is the etiologic agent of a granulocytotropic ehrlichiosis of humans, horses, and dogs. The DNA sequences of the 16S rDNA from the peripheral blood of naturally infected horses and dogs in Sweden, dogs in Minnesota and Wisconsin, and horses in Connecticut are identical with the HGE and differ slightly from the published 16S rDNA sequence for E. equi (2,54,55, and J. E. Madigan, J. E. Barlough, J. S. Dumler, N. S. Schankman, E. DeRock, unpublished observations). Moreover, when infected human blood from HGE patients is injected into horses, HGE develops, can be serially transmitted to other horses, and induces protection against subsequent E. equi challenge (56). Also, naturally occurring canine E. equi infections have been transmitted to horses that had developed equine granulocytic ehrlichiosis, and E. equi has been transmitted experimentally by injecting equine blood into susceptible dogs (57,58). Reproducible, continuous in vitro cultivation of all species of granulocytotropic ehrlichiae, including E. ewingii and E. equi, is an achievable goal.
E. equi antigens harvested from the blood of horses with high levels of parasitemia have been examined for reactivity with convalescent-phase human (HGE), equine (E. equi), canine (E. equi) and bovine (E. phagocytophila) sera by Western immunoblotting (59). The antigens judged most specific for E. equi were the 100-, 44-, 42-, and 25 kDa-bands. The most specific antigen, the 44-kDa band, was strongly reactive with the convalescent-phase human, equine, canine, and bovine sera, suggesting a specific antigenic relationship among these organisms from different animals and geographic origins. On the other hand, biologic studies of E. phagocytophila in Finland have shown apparent strain differences in virulence and lack of immunity to heterologous challenge (60). The molecular basis for these differences and the actual taxonomic relationships of HGE, E. equi, and E. phagocytophila remain to be determined.
Clinical Manifestations
A study of 41 cases of laboratory-confirmed human granulocytic ehrlichiosis from Minnesota and Wisconsin showed male predominance (78%), a median age of 59 years (range 6 to 91 years), median incubation period of 8 days, median period of fever and other symptoms before initiation of effective 5-day treatment, and year-round occurrence with a peak in June and July (61). Clinical manifestations included fever (100%), chills (98%), malaise (98%), myalgias (98%), headaches (85%), nausea (39%), vomiting (34%), cough (29%), confusion (17%), and rarely rash (2%). Four patients had pulmonary infiltrates visible on roentgenograms. The severity of illness is reflected in the rates of hospitalization (56%), admission to an intensive care unit (7%), and death (5%). The course of illness in patients who were not treated and yet survived included a 10-day febrile course in a child and 3- to 11-week remittent febrile course in adults (61).
Diagnosis and Treatment
A clinical diagnosis of human granulocytic ehrlichiosis should be considered in patients exposed to an Ixodes scapularis (dammini), I. pacificus or I. ricinus tick-infested environment who have a flulike febrile illness. Careful examination of a peripheral blood smear may show neutrophils that contain cytoplasmic vacuoles filled with ehrlichiae. Two different sets of PCR primers based upon the 16S rDNA sequence of HGE have been designed to amplify, detect, and identify HGE in the patient's blood during the acute stage of illness (2,62). Continued improvements in the technology are expected to yield highly sensitive and specific results in any well-managed clinical molecular diagnostics laboratory. Serologic diagnosis by IFA employing E. equi-infected neutrophils harvested from the blood of infected horses detects antibodies at a diagnostic titer of 80 or greater in the convalescent-phase sera of 100% of patients, but antibodies are usually not present in sera collected early in the illness.
Among 34 patients treated with doxycycline, 97% defervesced within 2 days. One patient who had not been treated with doxycycline had E. equi detectable by PCR in the blood 28 days after onset of symptoms.
Pathology, Pathogenesis, and Immunity
Following presumed injection of HGE into the patient's skin by the bite of Ixodes ssp. ticks, virtually none of the subsequent events are known. It is suspected that HGE infects a myeloid precursor in the bone marrow rather than mature neutrophils. Bone marrow examinations demonstrated hypercellularity in two patients and normocellular marrow in another patient. Autopsies of three patients who died of HGE showed opportunistic fungal pneumonia caused by a different agent (Aspergillus fumigatus, Cryptococcus neoformans, and Candida albicans) in each patient, suggesting altered host defenses (7,63). One of the patients who died had severe herpes esophagitis. Co-infection with HGE and B. burgdorferi is suspected to result in more severe disease. Examples from veterinary research indicating that E. phagocytophila and E. equi suppress the host defenses include opportunistic viral and fungal infections, decreased neutrophil adherence, emigration, phagocytosis, and bacterial killing, decreased production of antibodies, and decreased lymphocyte mitogenesis (64,65). The mechanisms by which ehrlichiae impair the host phagocytic and immune responses are not known.
Epidemiology and Ecology
Human granulocytic ehrlichiosis has been diagnosed in patients in Minnesota, Wisconsin, Massachusetts, Connecticut, New York, Rhode Island, Pennsylvania, Maryland, Florida, Arkansas, and California (7,63,66,67). Serologic evidence suggests that HGE or an antigenically related organism has also infected patients with Lyme borreliosis in Switzerland (68). An organism apparently identical to HGE is present in Sweden (54). There is compelling evidence that I. pacificus, the vector of Lyme borreliosis in northern California, transmits E. equi to horses (69). Ten percent of I. scapularis (dammini) ticks collected from vegetation in northwestern Wisconsin in 1982 and 1991 were infected with HGE, including two specimens containing both HGE and Borrelia burgdorferi. An engorged I. scapularis (dammini) tick was removed from a patient with human granulocytic ehrlichiosis in the same geographic area. PCR showed that the tick's salivary glands contained DNA of HGE (62). Similarly, PCR-amplified HGE DNA was detected in 50% of I. scapularis (dammini) ticks collected in Connecticut; no E. chaffeensis was detected in these ticks (70). Although the demonstration by PCR that blood from a high proportion of deer in a study in Wisconsin contained HGE suggests that deer might be an important reservoir, the possibility of a rodent reservoir should also be investigated. Indeed, the illustrations in a 1938 Tyzzer article suggest that Microtus pennsylvanicus and Peromyscus leucopus are naturally infected with a granulocytotropic ehrlichia (71).
Dr. Walker, professor and chair, Department of Pathology, and director, Center for Tropical Diseases, University of Texas Medical Branch at Galveston, has focused his biomedical research on rickettsial diseases during the last 22 years. Particular efforts have been expended in his laboratory since 1988 on the study of the agents and diseases of human ehrlichial infections. His efforts have contributed to the characterization of Rickettsia japonica, Ehrlichia chaffeensis, and R. felis as agents of emerging infectious diseases. The agent causing human granulocytic ehrlichiosis was first identified in his laboratory in Texas.
Dr. Dumler, assistant professor of pathology at the Johns Hopkins Medical Institutions, is the key scientist and physician in the detection, identification, and characterization of human granulocytic ehrlichiosis and its etiologic agent. A major contributor to the understanding of Ehrlichia chaffeensis, the human E. equi-like agent, and the diseases that they cause, Dr. Dumler has authored or coauthored 25 articles on these topics.
Acknowledgment
The authors express their gratitude to Dr. Vsevolod Popov for providing the excellent electron micrographs and to Ms. Josie Ramirez and Ms. Kay Kantowski for expert secretarial assistance in preparing the manuscript. This research was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI31431).
References
- Anderson BE, Dawson JE, Jones DC, Wilson KH. J Clin Microbiol. 1991;29:2838–42. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis.PubMedGoogle Scholar
- Chen S-M, Dumler JS, Bakken JS, Walker DH. J Clin Microbiol. 1994;32:589–95. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease.PubMedGoogle Scholar
- Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH, J Clin Microbiol. 1991;29:2741–5.Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis.PubMedGoogle Scholar
- Dumler JS, Chen S-M, Asanovich K, Trigiani E, Popov VL, Walker DH. J Clin Microbiol. 1995;33:1704–11. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosisPubMedGoogle Scholar
- Chen S-M, Popov VL, Feng H-M, Wen J, Walker DH. Infect Immun. 1995;63:647–55. Cultivation of Ehrlichia chaffeensis in mouse embryo, Vero, BGM, and L929 cells and study of Ehrlichia-induced cytopathic effect and plaque formationPubMedGoogle Scholar
- Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. N Engl J Med. 1987;316:853–6. Human infection with Ehrlichia canis, a leukocytic rickettsiaPubMedGoogle Scholar
- Bakken JS, Dumler JS, Chen SM, Eckman MR, Van Etta LL, Walker DH. human granulocytic ehrlichiosis in the upper midwest United States: a new species emerging? JAMA. 1994;272:212–8. DOIPubMedGoogle Scholar
- Anderson BE, Greene CE, Jones DA, Dawson JE. Int J Syst Bacteriol. 1992;42:299–302. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis.PubMedGoogle Scholar
- Wen B, Rikihisa Y, Mott J, Fuerst PA, Kawahara M, Suto C. Int J Syst Bacteriol. 1995;45:250–4. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics.PubMedGoogle Scholar
- Pretzman C, Ralph D, Stothard DR, Fuerst PA, Rikihisa Y. Int J Syst Bacteriol. 1995;45:207–11. 16S rRNA gene sequence of Neorickettsia helminthoeca and its phylogenetic alignment with members of the genus Ehrlichia.PubMedGoogle Scholar
- Ohashi N, Fukuhara M, Shimada M, Tamura A. FEMS Microbiol Lett. 1995;125:299–304. Phylogenetic position of Rickettsia tsutsugamushi and the relationship among its antigenic variants by analyses of 16S rRNA gene sequences. DOIPubMedGoogle Scholar
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308.PubMedGoogle Scholar
- Eng TR, Harkess JR, Fishbein DB, Dawson JE, Greene CN, Redus MA, Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA. 1990;264:2251–8. DOIPubMedGoogle Scholar
- Harkess JR, Ewing SA, Crutcher JM, Kudlac J, McKee G, Istre GR. Human ehrlichiosis in Oklahoma. J Infect Dis. 1989;159:576–9.PubMedGoogle Scholar
- Fishbein DB, Kemp A, Dawson JE, Greene NR, Redus MA, Fields DH. Human ehrlichiosis: prospective active surveillance in febrile hospitalized patients. J Infect Dis. 1989;160:803–9.PubMedGoogle Scholar
- Yevich SJ, Sanchez JL, DeFraites RF, Rives CC, Dawson JE, Uhaa IJ, Seroepidemiology of infections due to spotted fever group Rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J Infect Dis. 1995;171:1266–73.PubMedGoogle Scholar
- Dawson JE, Fishbein DB, Eng TR, Redus MA, Greene NR. Diagnosis of human ehrlichiosis with the indirect fluorescent antibody test: kinetics and specificity. J Infect Dis. 1990;162:91–5.PubMedGoogle Scholar
- Popov VL, Chen S-M, Feng H-M, Walker DH. Ultrastructural variation of Ehrlichia chaffeensis in cell culture. J Med Microbiol. In press.
- Silverman DJ, Wisseman CL Jr Comparative ultrastructural study on the cell envelopes of Rickettsia prowazekii, Rickettsia rickettsii, and Rickettsia tsutsugamushi.. Infect Immun. 1978;21:1020–3.PubMedGoogle Scholar
- Tamura A, Ohashi N, Urakami H, Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45:589–91.PubMedGoogle Scholar
- Chen SM, Dumler JS, Feng H-M, Walker DH. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1994;50:52–8.PubMedGoogle Scholar
- Sumner JW, Sims KG, Jones DC, Anderson BE. Infect Immun. 1993;61:3536–9. Ehrlichia chaffeensis expresses an immunoreactive protein homologous to the Escherichia coli GroEL protein.PubMedGoogle Scholar
- Fishbein DB, Dawson JE, Robinson LE. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–43.PubMedGoogle Scholar
- Fichtenbaum CJ, Peterson LR, Weil GJ. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am J Med. 1993;95:351–7. DOIPubMedGoogle Scholar
- Dumler JS, Sutker WL, Walker DH. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–5.PubMedGoogle Scholar
- Paddock CD, Suchard DP, Grumbach KL, Hadley WK, Kerschmann RL, Abbey NW, Brief report: fatal seronegative ehrlichiosis in a patient with HIV infection. N Engl J Med. 1993;329:1164–7. DOIPubMedGoogle Scholar
- Yu X, Brouqui P, Dumler JS, Raoult D. J Clin Microbiol. 1993;31:3284–8. Detection of Ehrlichia chaffeensis in human tissue by using a species-specific monoclonal antibody.PubMedGoogle Scholar
- Dumler JS, Brouqui P, Aronson J, Taylor JP, Walker DH. Identification of Ehrlichia in human tissue. N Engl J Med. 1991;325:1109–10.PubMedGoogle Scholar
- Moskovitz M, Fadden R, Min T. Human ehrlichiosis: a rickettsial disease associated with severe cholestasis and multisystemic disease. J Clin Gastroenterol. 1991;13:86–90. DOIPubMedGoogle Scholar
- Dunn BE, Monson TP, Dumler JS, Morris CC, Westbrook AB, Duncan JL, J Clin Microbiol. 1992;30:2207–10. Identification of Ehrlichia chaffeensis morulae in cerebrospinal fluid mononuclear cells.PubMedGoogle Scholar
- Everett ED, Evans KA, Henry RB, McDonald G. Human ehrlichiosis in adults after tick exposure: diagnosis using polymerase chain reaction. Ann Intern Med. 1994;120:730–5.PubMedGoogle Scholar
- Williams JD, Snow RM, Arciniegas JG. Myocardial involvement in a patient with human ehrlichiosis. Am J Med. 1995;98:414–5. DOIPubMedGoogle Scholar
- Standaert SM, Dawson JE, Schaffner W, Childs JE, Biggie KL, Singleton J Jr, N Engl J Med. 1995;333:420–5. Ehrlichiosis in a golf-oriented retirement community. DOIPubMedGoogle Scholar
- Anderson BE, Sumner JW, Dawson JE, Tzianabos T, Greene CR, Olson JG, J Clin Microbiol. 1992;30:775–80. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction.PubMedGoogle Scholar
- Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–44.PubMedGoogle Scholar
- Morais JD, Dawson JE, Greene C, Filipe AR, Galhardas LC, Bacellar F. First European case of ehrlichiosis. Lancet. 1991;338:633–4.PubMedGoogle Scholar
- Uhaa IJ, Maclean JD, Greene CR, Fishbein DB. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46:161–4.PubMedGoogle Scholar
- Dawson JE, Childs JE, Biggie KL, Moore C, Stallknecht D, Shaddock J, White-tailed deer as a potential reservoir of Ehrlichia spp. J Wildl Dis. 1994;30:162–8.PubMedGoogle Scholar
- Dawson JE, Strallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, J Clin Microbiol. 1994;32:2725–8. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis.PubMedGoogle Scholar
- Lockhart JM, Davidson WR, Dawson JE, Stallknecht DE. Temporal association of Amblyomma americanum with the presence of Ehrlichia chaffeensis reactive antibodies in white-tailed deer. J Wildl Dis. 1995;31:119–24.PubMedGoogle Scholar
- Dawson JE, Ewing SA. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322–7.PubMedGoogle Scholar
- Groves MG, Dennis GL, Amyx HL, Huxsoll DL. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res. 1975;36:937–40.PubMedGoogle Scholar
- Smith RD, Sells DM, Stephenson EH, Ristic M, Huxsoll DL. Development of Ehrlichia canis, causative agent of canine ehrlichiosis, in the tick Rhipicephalus sanguineus and its differentiation from a symbiotic rickettsia. Am J Vet Res. 1976;37:119–26.PubMedGoogle Scholar
- Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, J Med Entomol. 1995;32:368–74. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae).PubMedGoogle Scholar
- Roland WE, McDonald G, Caldwell CW, Everett ED. Ehrlichiosis—a cause of prolonged fever. Clin Infect Dis. 1995;20:821–5.PubMedGoogle Scholar
- Dumler JS, Dawson JE, Walker DH. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum Pathol. 1993;24:391–6. DOIPubMedGoogle Scholar
- Brouqui P, Raoult D. Antimicrob Agents Chemother. 1992;36:2799–803. In vitro antibiotic susceptibility of the newly recognized agent of ehrlichiosis in humans, Ehrlichia chaffeensis.PubMedGoogle Scholar
- Barnewall RE, Rikihisa Y. Infect Immun. 1994;62:4804–10. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron transferrin.PubMedGoogle Scholar
- Lewis GE Jr, Hill SL, Ristic M. Effect of canine immune serum on the growth of Ehrlichia canis within nonimmune canine macrophages. Am J Vet Res. 1978;39:71–6.PubMedGoogle Scholar
- Lewis GE Jr, Hill SL, Ristic M. Effect of canine immune macrophages and canine immune serum on the growth of Ehrlichia canis. Am J Vet Res. 1978;39:77–82.PubMedGoogle Scholar
- Caldwell CW, Everett ED, McDonald G, Yesus YW, Roland WE. Lymphocytosis of gamma/delta T cells in human ehrlichiosis. Am J Clin Pathol. 1995;103:761–6.PubMedGoogle Scholar
- Wilson KH, Blitchington RB, Greene RC. J Clin Microbiol. 1990;28:1942–6. Amplification of bacterial 16S ribosomal DNA and polymerase chain reaction.PubMedGoogle Scholar
- Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LSN. Engl. J Med. 1990;323:1573–80. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens.
- Johansson K-E, Pettersson B, Uhlen M, Gunnarsson A, Malmqvist M, Olsson E. Res Vet Sci. 1995;58:109–12. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from the 16S rRNA gene. DOIPubMedGoogle Scholar
- Greig B, Asanovich KM, Armstrong J, Dumler JS. Geographic, clinical, serologic and molecular findings of granulocytic ehrlichiosis in Minnesota and Wisconsin dogs, a likely zoonotic disease. J Clin Microbiol. In press.
- Madigan JE, Richter PJ Jr, Kimsey RB, Barlough JE, Bakken JS, Dumler JS. J Infect Dis. 1995;172:1141–4. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis.PubMedGoogle Scholar
- Lewis GE, Huxsoll DL, Ristic M, Johnson AJ. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. Am J Vet Res. 1975;36:85–8.PubMedGoogle Scholar
- Madewell BR, Gribble DH. Infection in two dogs with an agent resembling Ehrlichia equi. J Am Vet Med Assoc. 1982;180:512–4.PubMedGoogle Scholar
- Dumler JS, Asanovich KM, Bakken JS, Richter P, Kimsey R, Madigan JE. J Clin Microbiol. 1995;33:1098–103. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic ehrlichia.PubMedGoogle Scholar
- Tuomi J. Experimental studies on bovine tick-borne fever. 2. Differences in virulence of strains in cattle and sheep. Acta Pathol Microbiol Scand. 1967;70:577–89.PubMedGoogle Scholar
- Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Human granulocytic ehrlichiosis (HGE): clinical and laboratory characteristics of 41 patients from Minnesota and Wisconsin. JAMA. 1995;275:199–205. DOIGoogle Scholar
- Pancholi P, Kolbert CP, Mitchell PD, Reed KD, Dumler JS, Bakken JS, J Infect Dis. 1995;172:1007–12. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis.PubMedGoogle Scholar
- Hardalo CJ, Quagliarello V, Dumler JS. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–4.PubMedGoogle Scholar
- Larsen HJS, Overnes G, Waldeland H, Johansen GM. Immunosuppression in sheep experimentally infected with Ehrlichia phagocytophila. Res Vet Sci. 1994;56:216–24.PubMedGoogle Scholar
- Woldehiwet Z. The effects of tick-borne fever on some functions of polymorphonuclear cells of sheep. J Comp Pathol. 1987;97:481–5. DOIPubMedGoogle Scholar
- Telford III Sr, Lepore TJ, Snow P, Warner CK, Dawson JE. Human granulocytic ehrlichiosis in Massachusetts. Ann Intern Med. 1995;123:277–9.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Human granulocytic ehrlichiosis—New York, 1995. MMWR. 1995;44:593–5.PubMedGoogle Scholar
- Brouqui P, Dumler JS, Lienhard R, Brossard M, Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet. 1995;346:782–3. DOIPubMedGoogle Scholar
- Richter PJ, Kimsey RB, Madigan JE, Barlough JE, Dumler JS, Brooks DL. Ixodes pacificus as a vector of Ehrlichia equi. J Med Entomol. 1996;33:1–5.PubMedGoogle Scholar
- Magnarelli LA, Stafford KC III, Mather TN, Yeh M-T, Horn KD, Dumler JS. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–4.PubMedGoogle Scholar
- Tyzzer EE. Cytœcetes microti, N.G., N.Sp., a parasite developing in granulocytes and infective for small rodents. Parasitology. 1938;30:242–60. DOIGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 2, Number 1—January 1996
| EID Search Options |
|---|
|
|
|
|
|
|