Volume 22, Number 3—March 2016
Online Report
Global Progress and Challenges in Implementing New Medications for Treating Multidrug-Resistant Tuberculosis
Suggested citation for this article
Abstract
Two new drugs—bedaquiline and delamanid—have recently been approved by stringent regulatory authorities to treat multidrug-resistant tuberculosis (TB) and recommended by the World Health Organization for use under defined programmatic conditions. Introducing the medications in TB programs worldwide has not kept pace with the need for these drugs. In response, the DR-TB STAT (Drug-Resistant TB Scale-up Treatment Action Team) task force was formed in April 2015 to monitor progress and help overcome challenges. Information was collected from multiple sources and assessed monthly. Some progress has been made in introducing bedaquiline: as of October 2015, a total of 1,258 persons were on the medication under programmatic conditions. For delamanid, >100 patients, but few under programmatic conditions, have received the medication. Coordinated global action might help assist making these medications accessible for persons who need them most.
Multidrug-resistant (MDR) tuberculosis (TB) is a serious global public health problem (1), and estimates indicate that unless the management of MDR TB changes radically, it will be one of the main drivers of antimicrobial resistance, which could kill more persons than cancer by 2050 (2). Current therapy for MDR TB requires the use of multiple, toxic, and expensive drugs for 18–24 months and results in success in only about half of treated patients (3). The introduction of 2 new medications to treat MDR TB—bedaquiline (BDQ) and delamanid (DLM)—has renewed hope for improved individual outcomes and for stopping the ongoing transmission of MDR TB (4).
BDQ is a diarylquinoline agent (5) of Janssen Therapeutics (Titusville, NJ, USA) that, in December 2012, the US Food and Drug Administration conditionally recommended to treat MDR TB (6) after the results of a phase IIB randomized controlled trial showed faster time to culture conversion and higher rates of culture conversion at 6 months in patients who received the drug plus background regimen than in patients who received background regimen plus placebo (7). In June 2013, the World Health Organization (WHO) recommended BDQ for programmatic management of MDR TB when other alternatives are not adequate (8). DLM is a nitroimidazole agent (9) of Otsuka Pharmaceuticals (Princeton, NJ, USA) that, in April 2014, was granted conditional marketing authorization for treating MDR TB by the European Medicines Association (10) after a phase IIB trial with an open-label extension showed higher rates of culture conversion for persons receiving >2 months of DLM than for those receiving <2 months of the drug (11). In October 2014, WHO recommended use of DLM for treating MDR TB under specific conditions (12). WHO recommended both new drugs be used according to 5 criteria (Table 1). Although BDQ was generally recommended for patients with resistance or intolerance to the injectable agents, the fluoroquinolones, or both, DLM was additionally recommended for any MDR TB patient at high risk for poor treatment outcome.
Despite the conditional approval of these 2 new medications by stringent regulatory authorities and the WHO recommendations, BDQ and DLM have yet to reach most patients worldwide who have indications for these drugs (13). The high death rates for MDR TB and the ongoing spread of the disease in communities (14)—especially those with high rates of HIV (15)—prompted >88 civil society groups to issue an open letter calling for more urgent action to make BDQ and DLM available globally (16). This letter outlined several key aspirational and time-bound targets to be achieved by the letter’s addressees (Table 2). In response, a discussion was held in Geneva as part of a planned Global Laboratory Initiative/Global Drug Resistance Initiative (GDI) meeting, and a task force of the GDI (DR-TB STAT [Drug-Resistant TB Scale-up Treatment Action Team]) was formed (17). The goal of DR-TB STAT is to monitor progress against set time-bound targets for introducing BDQ and DLM under programmatic conditions for treating MDR TB. We present findings of the task force on global progress and challenges encountered in new drug introduction.
Task Force
The DR-TB STAT task force comprises stakeholders working to introduce new medications for treating MDR TB (Table 3). DR-TB STAT officially became a task force of the GDI in July 2015. Although task force status was pending, DR-TB STAT held its initial meeting in April 2015, and since then has met monthly by conference call to discuss progress and challenges in new drug introduction generally and in regard to specific countries and programs.
Information Collected
Information was collected from multiple sources: national TB programs, country reports, WHO reports, information from Janssen Therapeutics and Otsuka Pharmaceuticals, a variety of donors (US Agency for International Development [USAID], UNITAID, and The Global Fund), order data from the Global Drug Facility, and reports from nongovernment organizations (e.g., Partners in Health, Médecins Sans Frontières, Treatment Action Group, and KNCV Tuberculosis Foundation). Data were collected and updated monthly by using a standard collection form that included the following variables: 1) the number of patients treated with each drug as part of compassionate use/expanded access programs, 2) the number of patients treated with each drug under programmatic conditions, 3) the number of countries using each drug under programmatic conditions, 4) the number of orders placed for each drug, 5) the number of countries in which each drug has been registered or in which registration is pending, and 6) the projected number of patients and countries that will be receiving each drug in 2016. Summary data were tabulated to describe the overall assessment of global progress that is described here. For this report, compassionate use was defined as use of the drug accessed from the company for a specific patient; expanded access was defined as use of the drug accessed from the company for a group of patients who meet certain criteria (18).
In addition to focusing on global progress, DR-TB STAT also helps identify and address barriers to new drug introduction both generally and in program-specific settings. Detailed field notes documented the challenges reported by participants in the DR-TB STAT discussions. These notes were then reviewed to identify barriers to introduction of BDQ and DLM under programmatic conditions by using a thematic analysis (19). For the thematic analysis, notes were reviewed by a trained qualitative scientist (J.F.) to identify patterns and recurring content until saturation was reached. The responses were then grouped into topic areas.
BDQ
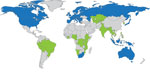
As of October 1, 2015, >700 persons had received BDQ through compassionate use and expanded access. In addition, 1,258 persons had received BDQ under programmatic conditions in 9 countries plus the European Union (EU). Orders for BDQ had been placed through the GDF for 1,680 persons in 24 additional countries, and these programmatic treatments are expected to begin in the next 6 months (Table 4; Figure 1).
In terms of registration, BDQ has been registered in the EU (28 countries) and 13 additional countries. Registration is pending in 13 other countries. Based on countries’ reported plans and the plans of other implementing groups, BDQ possibly could be given to a cumulative total of >7,000 persons under programmatic conditions by the end of 2016. Turnaround time from placement of order to drug delivery was 3–6 months
DLM
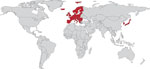
Despite repeated requests, Otsuka Pharmaceuticals did not provide specific information about DLM access, citing this information as proprietary. Therefore, data on the use of DLM were compiled from other sources. According to the company, as of October 1, 2015, >100 patients had received DLM through compassionate use. DLM orders cannot be placed through the GDF, and Otsuka Pharmaceuticals did not provide information about pending drug orders (Figure 2).
DLM has been registered in the EU, Japan, and South Korea. No information was found about pending registration in any additional countries. According to countries’ reported plans and the plans of other implementing groups, DLM could potentially be given to >550 persons under program conditions by the end of 2016. No information about turnaround time for drug orders could be obtained.
Data collected on barriers to use of new drugs that were discussed during DR-TB STAT meetings revealed a variety of reported challenges to using new drugs under programmatic conditions. In general, the problems reported fell into 10 areas: 1) lack of awareness of drug availability and procurement process; 2) limited availability of adequate technical expertise; 3) confusion around WHO “requirements,” most notably pharmacovigilance; 4) limited availability of quality clinical trials data supporting the use of new drugs under programmatic conditions; 5) challenges in sharing rapidly changing information about new drugs with key stakeholders and incorporating such information into national guidelines; 6) concerns that the process of new drug introduction is “too complicated” under programmatic conditions; 7) prolonged turnaround time for drug procurement; 8) difficulties in import and customs clearance; 9) limited access to companion MDR TB medications, especially linezolid and clofazimine; and 10) lack of high-level national government support.
Many of the goals toward achieving the targets in the global call to action have been met for BDQ. However, the goals set for DLM are unlikely to be met (Table 5).
Although MDR TB is treatable and curable, managing it under program conditions has long been characterized by a host of problems. These include challenges in the detection of resistance, especially to second-line drugs; delays in prompt initiation of appropriate therapy; and insufficient mechanisms for ongoing monitoring for toxicity and continued engagement of patients throughout the 18–24-month course of treatment. Furthermore, challenges exist to staff training and retention, information management, and infection control. Access to new drugs will not erase these barriers, but access does offer hope for improving individual patient outcomes and decreasing the transmission of MDR TB. Uptake of BDQ and DLM under programmatic conditions, however, has not kept pace with need. According to WHO recommendations for the use of these medications, 25%–50% of MDR TB patients meet criteria to receive these drugs because of their high-level resistance, intolerance to drugs currently used to treat MDR TB, or risks for poor outcomes (20). The occurrence of ≈500,000 new cases of MDR TB each year means that 125,000–250,000 new patients would benefit from the use of either of these drugs each year, a number that does not include the large number of prevalent MDR TB patients who might benefit from new treatment options. More conservative estimates of the need for new drugs using the actual numbers of MDR TB patients placed on treatment each year show that 24,250–48,500 of the 96,000 persons started on treatment (21) would benefit from access. Even using these less ambitious numbers, it is clear that more work needs to be done to improve access to BDQ and DLM.
The findings of DR-TB STAT show that more persons with MDR TB who have an indication for new drugs have access to BDQ than to DLM. Wider access to BDQ is occurring even though the WHO recommendations for DLM use are broader and despite the association of BDQ with a higher death rate in its phase IIB trial. For several reasons, access might be greater to BDQ than to DLM. BDQ was conditionally approved by a stringent regulatory authority and recommended by WHO >1 year before DLM was approved (22); over time, access to both drugs might become more comparable. However, several other possible explanations exist. First, the compassionate use/expanded access program for BDQ started nearly 3 years earlier and was more extensive and systematic than that for DLM. As a result, multiple countries and clinical providers have had experience with BDQ and thus might be more comfortable using it on a broader scale (23). Second, BDQ is registered in more countries than DLM; thus programmatic use might be more straightforward. Third, BDQ also is available through the GDF, whereas DLM can be obtained directly only from the company, although the company’s website contains no information about how the drug can be purchased (24). Finally, in March 2015, USAID began implementing a BDQ donation program in which 30,000 treatment courses are provided free of charge for 4 years, along with the technical assistance that countries might need to implement the drug (25). Although Otsuka Pharmaceuticals announced a “20 by 2020” access initiative—meaning 20% of MDR TB patients worldwide would receive DLM by 2020 (26)—the company has not provided information about how this program will work.
Stakeholders participating in the DR-TB STAT meetings discussed multiple barriers and challenges to new drug implementation that also appear to be slowing new drug introduction. Several of these seem to be misconceptions about how to access or use the drugs, including confusion about processes for procurement and import, the specific WHO recommendations—especially around pharmacovigilance—for the use of new drugs, and beliefs that the drugs cannot be used under programmatic conditions. Indeed, all stakeholders participating in DR-TB STAT listed lack of access to technical assistance from experienced providers, including provision of rapidly changing information about new drug introduction, as a major challenge. These challenges could be addressed by providing global training for programs and their implementing partners. One approach to filling this gap would be using innovative training tools, including Web-based training. Plans for offering such training globally are now being coordinated by the DR-TB STAT participants with the hope that much of the confusion about how to optimally use BDQ and DLM under programmatic conditions can be eased.
Other challenges in new drug implementation stem from lack of access to BDQ and DLM and the other drugs needed to form complete treatment regimens. Increased registration and additional experience importing the medications also should help the drugs clear customs more rapidly. The inclusion of both drugs, along with linezolid, on the WHO Model Essential Drugs List in April 2015 might help boost country confidence and registration for their use in additional countries.
Finally, improved access to companion drugs used with BDQ and DLM, especially linezolid and clofazimine, is urgently needed. Until sufficient evidence enables BDQ and DLM to be combined, linezolid and clofazimine (and other “group 5” drugs) are used to ensure that the new drugs are not added singly to a failing regimen. These companion drugs are expensive—in the case of linezolid, more so than BDQ—and are not registered with an MDR TB indication in most of the countries using BDQ and DLM. Similar attention needs to be given to these drugs, and countries need to be supported in accessing them. DR-TB STAT is likely to begin formally monitoring and assisting countries with access to these medications in 2016.
Access to new drugs alone will not solve the ongoing crisis of MDR TB, and much more comprehensive action is needed to improve care for persons with this disease on a global level (27). These medications, however, are one of the few current hopes for more effective and less toxic therapy for MDR TB–affected persons worldwide (28). Coordinated efforts can help ensure timely and equitable access to BDQ and DLM for persons who need them most.
Dr. Furinis an infectious diseases specialist and medical anthropologist, a lecturer at Harvard Medical School, and a consultant for a number of global agencies working on the introduction of new drugs for the treatment of MDR TB. Her research interests include optimal management of drug-resistant TB under program conditions, drug-resistant TB in children, and use of new drugs for treating and preventing drug-resistant TB.
Acknowledgments
We are grateful to numerous colleagues whose efforts have made the work of the task force possible, including those from National TB Programs, the Stop TB Partnership, WHO, multiple nongovernment organizations, and the community of activists. Finally, we are mindful of the men and women who are living with, have survived, or have died from MDR TB and for whom access to these medications is most crucial.
The Global Drug-Resistance Initiative, USAID, and UNITAID funded the task force.
References
- Zumla AI, Schito M, Maeurer M. Advancing the portfolio of tuberculosis diagnostics, drugs, biomarkers and vaccines. Lancet Infect Dis. 2014;14:267–9. DOIPubMedGoogle Scholar
- Walsh J. Review on antimicrobial resistance: tackling drug-resistant infections. February 2015 [cited 2015 Dec 29]. http://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
- Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. DOIPubMedGoogle Scholar
- Field SK, Fisher D, Jarand JM, Cowie RL. New treatment options for multidrug-resistant tuberculosis. Ther Adv Respir Dis. 2012;6:255–68. DOIPubMedGoogle Scholar
- Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L. al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–405. DOIPubMedGoogle Scholar
- US Food and Drug Administration. FDA approval letter for Sirturo (bedaquiline). 2012 [cited 2015 Dec 29]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist
- Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371:723–32. DOIPubMedGoogle Scholar
- World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis. Geneva: The Organization; 2013.
- Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–60. DOIPubMedGoogle Scholar
- Business Wire. Otsuka wins European marketing authorization for Deltyba™ (delamanid) [cited 2015 Aug 25]. http://www.businesswire.com/news/home/20140429006457/en/Otsuka-Wins-European-Marketing-Authorization-Deltyba
- Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41:1393–400. DOIPubMedGoogle Scholar
- World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Geneva: The Organization; 2014.
- Lessem E, Cox H, Daniels C, Furin J, McKenna L, Mitnick CD, Access to new medications for the treatment of drug-resistant tuberculosis: patient, provider and community perspectives. Int J Infect Dis. 2015;32:56–60. DOIPubMedGoogle Scholar
- Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MMDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS ONE. 2015;10:e0119332. DOIPubMedGoogle Scholar
- Manda SO, Masenyetse LJ, Lancaster JL, van der Walt ML. Risk of death among HIV co-infected multidrug-resistant tuberculosis patients, compared to morality in the general population of South Africa. J AIDS Clin Res. 2013;(Jul 2)Suppl 3:7.
- Médecins Sans Frontières Access Campaign. Call to action: accelerate access to DR-TB drugs [cited 2015 Aug 25]. http://www.msfaccess.org/content/call-action-accelerate-access-dr-tb-drugs
- Global Laboratory Initiative/Global Drug-Resistance Initiative. Joint Partners Forum for strengthening and aligning TB diagnosis and treatment. Geneva, Switzerland. April 27–30, 2015 [cited 2015 Aug 25]. http://www.stoptb.org/wg/mdrtb/
- Horsburgh CR Jr, Haxaire-Theeuwes M, Lienhardt C, Wingfield C, McNeeley D, Pyne-Mercier L, Compassionate use of and expanded access to new drugs for drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2013;17:146–52. DOIPubMedGoogle Scholar
- Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15:398–405. DOIPubMedGoogle Scholar
- World Health Organization. Companion handbook to the 2011 WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: The Organization; 2014.
- World Health Organization. Global tuberculosis report 2014. Geneva: The Organization; 2014.
- World Health Organization. Introduction of bedaquiline for the treatment of multidrug-resistant tuberculosis at country level—implementation plan. WHO/HTM/TB/2015 [cited 2015 Aug 25]. http://www.who.int/tb/publications/WHO_BDQimplementationplan.pdf
- Ndjeka N, Conradie F, Schnippel K, Hughes J, Bantubani N, Ferreira H, Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015;19:979–85. DOIPubMedGoogle Scholar
- Otsuka Holdings Co. Ltd. Otuska: people creating new products for better health worldwide [cited 2015 Oct 14]. http://www.otsuka.com/en/
- Stop TB. Partnership. USAID bedaquiline donation program begins today [cited 2015 Aug 25]. http://www.stoptb.org/news/stories/2015/ns15_015.asp
- Wells CD. Delamanid for MDR‐TB: current development progress and ongoing access plans [cited 2015 Aug 25]. http://www.stoptb.org/wg/gli/assets/documents/M7/3.%20WELLS_Introduction%20to%20Delamanid.pdf
- Toczek A, Cox H, du Cros P, Cooke G, Ford N. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:299–307. DOIPubMedGoogle Scholar
- Brigden G, Nyang'wa BT, du Cros P, Varaine F, Hughes J, Rich M, Principles for designing future regimens for multidrug-resistant tuberculosis. Bull World Health Organ. 2014;92:68–74. DOIPubMedGoogle Scholar
Figures
Tables
Suggested citation for this article: Furin J, Brigden G, Lessem E, Rich M, Vaughan L, Lynch S. Global progress and challenges in implementing mew medications for treating multidrug-resistant tuberculosis. Emerg Infect Dis. 2016 Mar [date cited]. http://dx.doi.org/10.3203/eid2203.151430
Table of Contents – Volume 22, Number 3—March 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jennifer Furin, Harvard Medical School, 641 Huntington Ave, Boston, MA 02115, USA
Top