Volume 22, Number 6—June 2016
CME ACTIVITY - Synopsis
Infectious Disease Risk Associated with Contaminated Propofol Anesthesia, 1989–20141
Figure 4
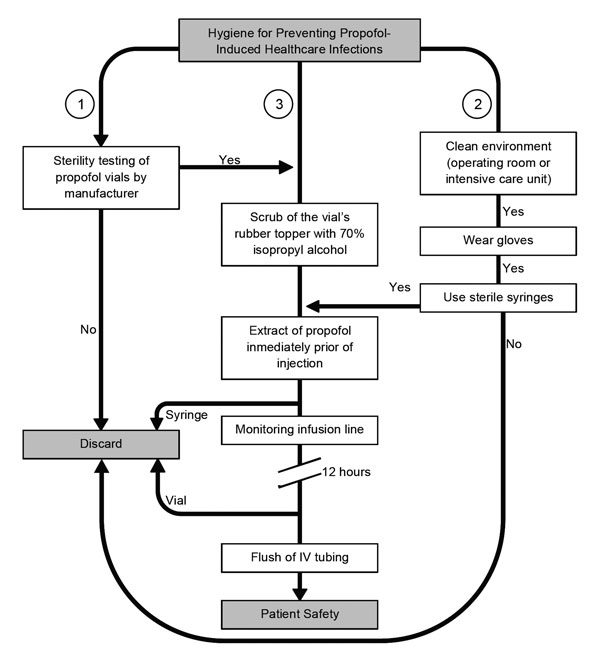
Figure 4. Algorithm for helping reduce the likelihood of infectious disease events when using propofol. To avoid intrinsic contamination, sufficient quality control during the manufacturers’ process is required (1). Personnel must be aware of the importance of performing healthcare procedures in a clean environment and the use of gloves and sterile syringes for anesthetic procedures. Syringes and needles must never be reused (2). Also, the aseptic technique for administration of propofol includes cleaning of the rubber bung, if present, with isopropyl alcohol, leaving it to dry. Propofol should be drawn up immediately before its use and not left standing. Intravenous (IV) infusion lines and stopcock dead spaces should be completely flushed to ensure no residual propofol remains. Vials must be discarded after opening for single use, no matter the amount of the remainder (3).
1Part of this work was presented at the XXXI Colombian Congress of Anesthesiology and Critical Care, Cali, Columbia, July 2015.