Volume 22, Number 8—August 2016
Research
Hemolysis after Oral Artemisinin Combination Therapy for Uncomplicated Plasmodium falciparum Malaria
Figure 2
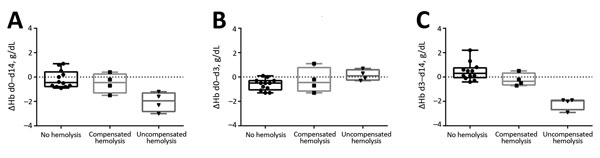
Figure 2. Changes in hemoglobin levels (ΔHb) for patients without posttreatment hemolysis, with compensated posttreatment hemolysis, and with uncompensated posttreatment hemolysis after treatment with oral artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria. A) day (d) 0 to d 14 (overall); B) d 0 to d 3 (treatment period); C) d 3 to d 14 (posttreatment period). Horizontal lines indicate median values, boxes indicate interquartile ranges, whiskers indicate ranges, and solid squares, circles, and triangles indicate individual patient data points.
Page created: July 15, 2016
Page updated: July 15, 2016
Page reviewed: July 15, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.