Volume 22, Number 9—September 2016
Letter
Vibrio cholerae O1 Imported from Iraq to Kuwait, 2015
Cite This Article
Citation for Media
To the Editor: The etiologic agent of the sixth pandemic of cholera was classical biotype of Vibrio cholerae O1. The ongoing seventh pandemic is caused by El Tor biotype. The biotypes are differentiated by phenotypic and genotypic characteristics. However, this differentiation blurred when V. cholerae O1 strains were detected in Matlab, Bangladesh, in 2006, in which characteristics were mixed. Genetically, the differences occurred in tcpA, which encodes the major adherence antigen rstR that regulates site-specific recombination of CTXϕ phage and ctxB that encodes the B subunit of cholera toxin. These genes had the characteristics of classical biotype in Matlab variants of El Tor strains. Later, various types of El Tor variants were reported in Southeast Asia, Africa, and Haiti. Differentiating features also occur in repeat toxin A gene (rtxA), chromosomal location of CTXϕ, the number of heptad repeats in ToxR binding region, and the occurrence of vibrio seventh pandemic islands I and II (1,2).
Kuwait is free of endemic cholera, but imported cases occur there (3). Cholera is endemic to neighboring Iraq. An outbreak caused by V. cholerae O1 Inaba serotype started in Iraq in September 2015 (4). However, a full characterization of the strain is lacking. A thorough characterization of the strain assumes urgency in light of the spread of variants. We characterized isolates from 2 recent cholera cases imported to Kuwait from Iraq.
The first case was in a 19-year-old Kuwaiti man who visited Najaf and Karbala in Iraq in September 2015; the second case was in a 52-year-old Kuwaiti woman who visited the same 2 locations in October 2015. Both had watery diarrhea 3–4 times daily and vomiting; they returned to Kuwait and were admitted to Al Amiri Hospital (Sharq, Kuwait). They gave histories of drinking local water in Iraq, had moderate dehydration, and were treated with intravenous rehydration solution and a single doxycycline dose (500 mg). Diarrhea resolved after 2–3 days.
Fecal specimens collected at admission from both patients grew yellow colonies on thiosulfate bile salt sucrose agar (Eiken, Tokyo, Japan); these colonies were confirmed as V. cholerae O1 Inaba serotype by biochemical reactions and agglutination with specific antiserum (Denka Seiken, Tokyo, Japan). The woman’s isolate was designated as Kuwait 36 and the man’s as Kuwait 37. The isolates were positive for chicken cell agglutination and Voges-Proskauer tests and were polymyxin B resistant, characteristics of El Tor biotype. The isolates were resistant to nalidixic acid but susceptible to ciprofloxacin, norfloxacin, ofloxacin, tetracycline, meropenem, ampicillin, ceftriaxone, trimethoprim/sulfamethoxazole, chloramphenicol, erythromycin, azithromycin, streptomycin, neomycin, and gentamicin by disk diffusion test. Tetracycline susceptibility confirmed favorable response to doxycycline.
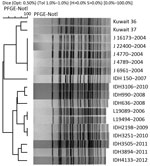
We studied the genotype of ctxB using a double-mismatch amplification mutation PCR (i.e., mismatches in both primers). PCR with classical ctxB-specific primers ctxBF4/ctxBRvCla yielded an amplicon of 191 bp, but not with Haitian ctxB specific primers ctxBF3/ctxBRvCla, indicating that the isolates had a ctxB of classical biotype (genotype 1) (5,6). Mismatch amplification mutation assay PCR (MAMA-PCR, i.e., mismatch in only 1 primer) with Haitian-specific tcpA primers tcpAF2/tcpARev produced an amplicon of 167 bp but not with El Tor tcpA–specific primers tcpAF1/tcpAElRev, suggesting these isolates had the Haitian variant tcpA (2). MAMA-PCR for rtxA with El Tor–specific primer pair rtxAF/rtxAR1 yielded a 187-bp amplicon but no amplicon for Haitian variant primer pair rtxAF/rtxAR2, suggesting the occurrence of rtxA of El Tor variety (2). The isolates possessed El Tor type rstR because they produced a 500-bp amplicon with primer pair rstR2/rstA3R (7). The isolates were positive for rstC, a repeat sequence activator found in El Tor biotype, because they yielded an amplicon of 238 bp with primer pair rstC1/rstC2 (8). rstB is required for CTXϕ phage integration. The Haitian strain has a GTA deletion at positions 77–79. MAMA-PCR with primer pair rstB F1/rstB R1 produced a 160-bp amplicon, suggesting the absence of deletion in El Tor type rstB (2). The isolates had CTXϕ integrated in the large chromosome with RS element downstream because they produced a 766-bp amplicon with CII F/CII R primers (9). PCR sequencing with primers Zot F/ctxA R indicated the presence of 4 heptad (TTTTGAT) repeats in the ToxR binding region of ctxAB promoter, similar to El Tor biotype (2). Both isolates possessed vibrio seventh pandemic islands I and II, typical of El Tor biotype as assessed by PCRs with a variety of primers (10). Clonal relationship studied by pulsed-field gel electrophoresis suggested that isolates from the Kuwaiti patients were similar to each other and closer to Indian isolates of 2004 (Figure). Cholera is endemic to India; many El Tor variants circulate there (2).
We showed that the strain causing cholera in Iraq did not have the typical El Tor characteristics but instead had mixed characteristics of El Tor, classical, and Haitian strains. Altered strains of V. cholerae O1 might have implications for disease severity and vaccine efficacy (1). El Tor variants seem to be sweeping the world. We wonder whether they could replace the archetypal El Tor strain and become the causative agent of the eighth pandemic of cholera.
Acknowledgment
This study was supported in part by the Indian Council of Medical Research, Government of India. P.S. received a Junior Research Fellowship from the Council of Scientific and Industrial Research, Government of India.
References
- Safa A, Nair GB, Kong RYC. Evolution of new variants of Vibrio cholerae O1. Trends Microbiol. 2010;18:46–54. DOIPubMedGoogle Scholar
- Ghosh P, Naha A, Pazhani GP, Ramamurthy T, Mukhopadhyay AK. Genetic traits of Vibrio cholerae O1 Haitian isolates that are absent in contemporary strains from Kolkata, India. PLoS One. 2014A;9:e112973. DOIPubMedGoogle Scholar
- Joshi RM, Albert MJ. Hybrid El Tor Vibrio cholerae O1, Kuwait. Emerg Infect Dis. 2009;15:1879–80. DOIPubMedGoogle Scholar
- Bagcchi S. Cholera in Iraq strains the fragile state. Lancet Infect Dis. 2016;16:24–5. DOIPubMedGoogle Scholar
- Morita M, Ohnishi M, Arakawa E, Yamamoto S, Nair GB, Matsushita S, Emergence and genetic diversity of El Tor Vibrio cholerae O1 that possess classical biotype ctxB among travel-associated cases of cholera in Japan. J Med Microbiol. 2010;59:708–12. DOIPubMedGoogle Scholar
- Naha A, Chowdhury G, Ghosh-Banerjee J, Senoh M, Takahashi T, Ley B, Molecular characterization of high-level-cholera-toxin–producing El Tor variant Vibrio cholerae strains in the Zanzibar Archipelago of Tanzania. J Clin Microbiol. 2013;51:1040–5. DOIPubMedGoogle Scholar
- Nusrin S, Khan GY, Bhuiyan NA, Ansaruzzaman M, Hossain MA, Safa A, Diverse CTX phages among toxigenic Vibrio cholerae O1 and O139 strains isolated between 1994 and 2002 in an area where cholera is endemic in Bangladesh. J Clin Microbiol. 2004;42:5854–6. DOIPubMedGoogle Scholar
- Waldor MK, Rubin EJ, Pearson GD, Kimsey H, Mekalanos JJ. Regulation, replication, and integration functions of the Vibrio cholerae CTXphi are encoded by region RS2. Mol Microbiol. 1997;24:917–26. DOIPubMedGoogle Scholar
- Maiti D, Das B, Saha A, Nandy RK, Nair GB, Bhadra RK. Genetic organization of pre-CTX and CTX prophages in the genome of an environmental Vibrio cholerae non-O1, non-O139 strain. Microbiology. 2006;152:3633–41. DOIPubMedGoogle Scholar
- O’Shea YA, Reen FJ, Quirke AM, Boyd EF. Evolutionary genetic analysis of the emergence of epidemic Vibrio cholerae isolates on the basis of comparative nucleotide sequence analysis and multilocus virulence gene profiles. J Clin Microbiol. 2004;42:4657–71. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 22, Number 9—September 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
M. John Albert, Department of Microbiology, Faculty of Medicine, Kuwait University, Jabriya, Kuwait; email.
Top