Volume 23, Supplement—December 2017
SUPPLEMENT ISSUE
Global Health Security Supplement
Prevent
CDC Activities for Improving Implementation of Human Papillomavirus Vaccination, Cervical Cancer Screening, and Surveillance Worldwide
Figure
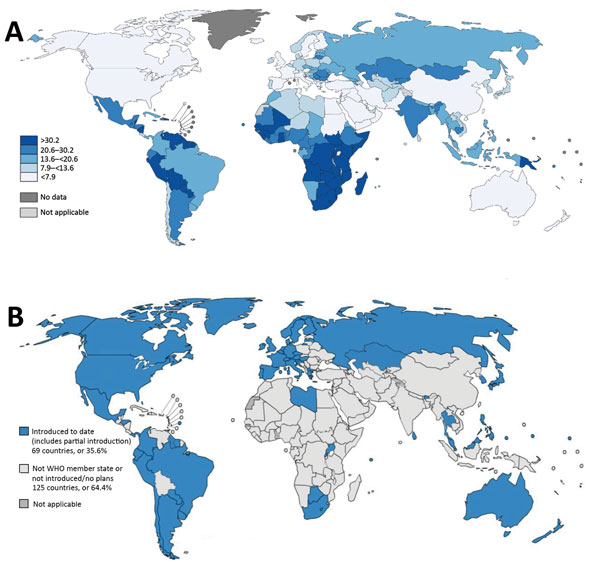
Figure. Worldwide cervical cancer incidence and human papillomavirus (HPV) vaccination status. A) Estimated cervical cancer incidence rates per 100,000 persons in 2012. Source: GLOBOCAN, 2012, WHO. B) Progress in HPV vaccine introduction in national immunization programs, 2016. Source: WHO, 2016. Many countries with high cervical cancer incidence rates (primarily countries in sub-Saharan Africa, Asia, and a few in Latin America) have not yet introduced HPV vaccination in their national immunization programs. Cervical cancer can also be prevented by screening and treatment for precancerous lesions; incidence and mortality rates in high-income countries have decreased largely because of effective screening programs. Data for cervical cancer screening coverage worldwide are limited; 2002 World Health Survey data showed that the proportion of women who had a Papanicolaou test in the previous 3 years greatly varied among countries; 11%–83% in industrialized countries, and 1%–73% in developing countries (5). WHO, World Health Organization.
References
- International Agency for Research on Cancer. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC Cancer Base No. 11, 2013. Lyon (France): International Agency for Research on Cancer 2014 [cited 2017 Sep 27]. http://publications.iarc.fr/Databases/Iarc-Cancerbases/Globocan-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1-0-2012
- Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–14. DOIPubMedGoogle Scholar
- Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al.; HPV Typing of Cancers Workgroup. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107:djv086. DOIPubMedGoogle Scholar
- Schiffman M, Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;31:14–9. DOIPubMedGoogle Scholar
- Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:e132. DOIPubMedGoogle Scholar
- International Agency for Research on Cancer. IARC handbooks of cancer prevention. Vol. 10 [cited 2017 Sep 27]. http://www.iarc.fr/en/publications/pdfs-online/prev/handbook10/
- World Health Organization. Immunization vaccines and biologicals database, April 2017. Geneva: The Organization; 2017.
- World Health Organization. Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention, 2013 [cited 2017 Sep 27]. http://www.who.int/reproductivehealth/publications/cancers/screening_and_treatment_of_precancerous_lesions/en/
- Council on Foreign Relations. The emerging global health crisis: noncommunicable diseases in low-and middle-income countries, 2014. Independent Task Force Report No. 72 [cited 2017 Sep 27]. http://www.cfr.org/diseases-noncommunicable/emerging-global-health-crisis/p33883
- Centers for Disease Control and Prevention. CDC’s strategic framework for global immunization, 2016–2020, 2016 [cited 2017 Sep 27]. https://www.cdc.gov/globalhealth/immunization/docs/global-immunization-framework-508.pdf
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec. 2009;84:118–31.PubMedGoogle Scholar
- World Health Organization. Report of the meeting on HPV vaccine coverage and impact monitoring, November 16–17, 2009. Geneva: The Organization; 2010 [cited 2017 Oct 19] http://apps.who.int/iris/bitstream/10665/70305/1/WHO_IVB_10.05_eng.pdf
- World Health Organization. Recommendations to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines. Geneva: The Organization; 2015 [cited 2017 Oct 19]. http://www.who.int/biologicals/IPV_FINAL_for_BS2233_07072014(2).pdf
- Gavi, The Vaccine Alliance. Human papillomavirus vaccine support, 2017 [cited 2017 Feb 15]. http://www.gavi.org/support/nvs/human-papillomavirus/
- PATH Publications and London School of Hygiene and Tropical Medicine. HPV vaccine lessons learned. RHO Cervical Cancer, 2014–2016 [cited 2017 Feb 15]. http://www.rho.org/HPVlessons/
- Meeting of the Strategic Advisory Group of Experts on Immunization. October 2016: conclusions and recommendations. Wkly Epidemiol Rec. 2016;91:561–82.PubMedGoogle Scholar
- Friedman AL, Oruko KO, Habel MA, Ford J, Kinsey J, Odhiambo F, et al. Preparing for human papillomavirus vaccine introduction in Kenya: implications from focus-group and interview discussions with caregivers and opinion leaders in Western Kenya. BMC Public Health. 2014;14:855. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Progress toward implementation of human papillomavirus vaccination—the Americas, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1382–4.PubMedGoogle Scholar
- Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, et al. Human papillomavirus vaccine introduction—the first five years. Vaccine. 2012;30(Suppl 5):F139–48. DOIPubMedGoogle Scholar
- Pan American Health Organization/World Health Organization/Centers for Disease Control and Prevention. Integrating HPV testing in cervical cancer screening programs: a manual for program managers, 2016 [cited 2017 Sep 27]. http://iris.paho.org/xmlui/handle/123456789/31393
- Joint United Nations Programme on HIV and AIDS. The George W. Bush Institute, the U.S. Department of State, Susan G. Komen for the Cure, and UNAIDS announce a new women’s health initiative (press release) [cited 2017 Sep 27]. http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2011/september/20110913pinkribbonredribbon
- Sangrajrang S, Laowahutanont P, Wongsena M, Muwonge R, Karalak A, Imsamran W, et al. Comparative accuracy of Pap smear and HPV screening in Ubon Ratchathani in Thailand. Papillomavirus Res. 2017;3:30–5. DOIPubMedGoogle Scholar
- Drummond JL, Were MC, Arrossi S, Wools-Kaloustian K. Cervical cancer data and data systems in limited-resource settings: Challenges and opportunities. Int J Gynaecol Obstet. 2017;138(Suppl 1):33–40. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. CDC Foundation and George W. Bush Institute partner in $3.6 million grant to address global cervical cancer. April 16, 2014 [cited 2017 Sep 27]. https://www.cdcfoundation.org/pr/2014/cdc-foundation-bush-institute-partnership-to-address-global-cervical-cancer
- Centers for Disease Control and Prevention Foundation. Improving data for decision-making in global cervical cancer programs (IDCCP). Accelerating action on human papillomavirus and cervical cancer prevention and control (APEC) Conference: Implementing Policy Recommendations Workshop; August 23, 2016; Lima, Peru [cited 2017 Sep 27]. http://mddb.apec.org/Documents/2016/HWG/HWG-LSIF-WKSP/16_hwg-lsif_wksp_006.pdf
- Joseph R, Manosoontorn S, Petcharoen N, Sangrajrang S, Senkomago V, Saraiya M. Assessing cervical cancer screening coverage using a population-based behavioral risk factor survey—Thailand, 2010. J Womens Health (Larchmt). 2015;24:966–8. DOIPubMedGoogle Scholar
- Wang B, He M, Chao A, Engelgau MM, Saraiya M, Wang L, et al. Cervical cancer screening among adult women in China, 2010. Oncologist. 2015;20:627–34. DOIPubMedGoogle Scholar
- Buchanan Lunsford N, Ragan K, Lee Smith J, Saraiya M, Aketch M. Environmental and psychosocial barriers to and benefits of cervical cancer screening in Kenya. Oncologist. 2017;22:173–81. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Field Epidemiology Training Program: where we work [cited 2017 Feb 17]. https://www.cdc.gov/globalhealth/healthprotection/fetp/where/index.html
- The Cancer Atlas. Reliable monitoring of cancer cases and deaths is essential for successful cancer management plans, 2017 [cited 2017 Sep 27]. http://canceratlas.cancer.org/taking-action/cancer-registries/
- International Agency for Research on Cancer (IARC). WHO global initiative for cancer registry development (GICR), 2017 [cited 2017 Sep 27]. http://gicr.iarc.fr/
- Saraiya M, Tangka FK, Asma S, Richardson LC. Importance of economic evaluation of cancer registration in the resource limited setting: Laying the groundwork for surveillance systems. Cancer Epidemiol. 2016;45(Suppl 1):S1–3. DOIPubMedGoogle Scholar