Volume 23, Number 3—March 2017
Research
Comparison of Sputum-Culture Conversion for Mycobacterium bovis and M. tuberculosis
Cite This Article
Citation for Media
Abstract
Current US guidelines recommend longer treatment for tuberculosis (TB) caused by pyrazinamide-resistant organisms (e.g., Mycobacterium bovis) than for M. tuberculosis TB. We compared treatment response times for patients with M. bovis TB and M. tuberculosis TB reported in the United States during 2006–2013. We included culture-positive, pulmonary TB patients with genotyping results who received standard 4-drug treatment at the time of diagnosis. Time to sputum-culture conversion was defined as time between treatment start date and date of first consistently culture-negative sputum. We analyzed 297 case-patients with M. bovis TB and 30,848 case-patients with M. tuberculosis TB. After 2 months of treatment, 71% of M. bovis and 65% of M. tuberculosis TB patients showed conversion of sputum cultures to negative. Likelihood of culture conversion was higher for M. bovis than for M. tuberculosis, even after controlling for treatment administration type, sex, and a composite indicator of bacillary burden.
The Mycobacterium tuberculosis complex is composed of several genetically related and pathogenic mycobacterial species, including M. tuberculosis and M. bovis (1). Tuberculosis (TB) caused by these species is often clinically indistinguishable, although M. bovis has a different epidemiologic profile (2–5). Despite similarities, there is growing evidence that diversity within the M. tuberculosis complex has major immunologic consequences, which may influence treatment response (6–11). Sputum-culture conversion (i.e., conversion from positive to negative culture result) is considered the principal prognostic indicator for treatment response and is often used as a surrogate endpoint in early-phase randomized clinical trials (12,13). Studies have demonstrated that sputum-culture conversion differs by M. tuberculosis phylogenetic lineage (14,15).
M. bovis is generally considered intrinsically resistant to pyrazinamide, which is considered an essential first-line anti-TB drug. Pyrazinamide is a sterilizing drug that acts synergistically with rifampin to shorten the duration of anti-TB treatment from 9 to 6 months (16). In the absence of this benefit, many experts currently recommend extending treatment for TB caused by M. bovis (17). However, these recommendations are based on expert opinion and lack definitive evidence from laboratory studies or randomized clinical trials (17). We evaluated differences in time from treatment initiation to sputum-culture conversion between patients with M. bovis TB and M. tuberculosis TB given standard first-line anti-TB treatment.
We analyzed data from the National Tuberculosis Surveillance System (NTSS) at the Centers for Disease Control and Prevention (Atlanta, GA, USA) and restricted analysis to cases reported during 2006–2013 to permit sufficient time for follow-up reporting of outcome data. M. tuberculosis complex isolates were identified by using spoligotyping and multilocus variable number tandem repeat (i.e., mycobacterial interspersed repetitive unit−variable number tandem repeat) genotyping techniques (3,18).
We used a retrospective cohort study design and included culture-confirmed TB cases with complete genotyping results and pulmonary disease treated with a standard 4-drug regimen (i.e., isoniazid, rifampin, ethambutol, and pyrazinamide) at diagnosis (19). Cases with isolates identified as any species other than M. tuberculosis or M. bovis were excluded; cases with isolates identified as M. bovis Bacillus Calmette–Guérin were assumed to be iatrogenic (20) and were also excluded. We excluded from analysis any case-patients with organisms initially resistant to rifampin or isoniazid, those infected with M. tuberculosis initially resistant to pyrazinamide, those who were dead at time of diagnosis, and those with missing or unreliable culture-conversion data. We used the Pearson χ2 test to compare clinical and demographic characteristics of case-patients with M. bovis and M. tuberculosis TB and the proportion of case-patients who showed conversion of cultures at 2 and 3 months.
Time to sputum-culture conversion was calculated for persons with positive sputum cultures as the number of days from the date treatment started until the date of the first consistently culture-negative sputum. The date of the first consistently culture-negative sputum was defined as the date that a specimen was collected for the first documented negative culture result with no concurrent (i.e., samples collected within 1 week) or subsequent positive cultures. We used a Kaplan-Meier estimator to calculate survival-like function curves for time to sputum-culture conversion for persons with M. bovis and M. tuberculosis TB.
We censored, at the date recorded for each outcome, patients who died during treatment and those who moved, were lost to follow-up, or otherwise stopped treatment before the expected completion date; we restricted the analysis to the first 90 days of anti-TB treatment. We used Cox proportional hazard modeling to calculate adjusted hazard ratios (aHRs) with 95% CIs of factors associated with time to culture conversion. We tested the proportional hazards assumption by graphing log (–log [survival probability]) versus log (time) for all covariates of interest. We assessed a priori covariates of interest, including sex; treatment administration type (directly observed therapy versus self-administered therapy); reported HIV status (positive, negative, and unknown); sputum smear status (positive, negative, not obtained, and unknown); and cavitary disease found by diagnostic imaging (chest radiography or computed tomography; positive, negative, not done, and unknown) results.
After reviewing smear status and diagnostic imaging results, we created a composite variable for bacillary burden (high, medium, low, and unknown) to avoid collinearity. We categorized cases with confirmed cavitary disease identified by imaging and positive sputum smear as high bacillary burden, cases with either confirmed cavitary disease or positive sputum smear as medium bacillary burden, cases with no cavitary disease and negative sputum smears as low bacillary burden, and cases without any of these indicators as unknown bacillary burden. In the time-to-event analysis, we excluded patients who had unknown bacillary burden. Analysis was conducted by using SAS version 9.3 (SAS Institute, Cary, NC, USA).
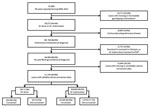
A total of 91,985 TB cases during 2006–2013 were available for analysis (Figure 1). Approximately two thirds (64.4%) of these cases had complete genotyping results and were identified as either M. tuberculosis TB or M. bovis TB. All cases of TB with initial resistance to isoniazid or rifampin (n = 4,418) and M. tuberculosis TB cases with any initial resistance to pyrazinamide (n = 757) were excluded. The final dataset for analysis included 297 cases of M. bovis TB and 30,848 cases of M. tuberculosis TB. All covariates met the proportional hazards assumption except for HIV status, which was excluded from further analysis because of sparse data.
The age distributions of patients given a diagnosis of M. bovis TB and patients given a diagnosis of M. tuberculosis TB were similar (Table 1). A greater proportion of patients with M. bovis TB were female (123/297 [41.1%]) than patients with M. tuberculosis TB (10,536/30,848 [34.2%]; p = 0.01). Patients with M. bovis TB were most often born outside the United States (86.2%), and most self-identified as Hispanic (93.2%). In comparison, just over half (59.5%) of M. tuberculosis TB patients were born outside the United States; 30.3% self-identified as Hispanic (p<0.0001 for both comparisons).
Approximately one third (35.0%) of M. bovis TB patients had pulmonary and extrapulmonary involvement at diagnosis compared with 10.0% of M. tuberculosis TB patients (p<0.0001). Similar proportions of patients had cavitary chest lesions documented (M. bovis TB = 46.6%, M. tuberculosis TB = 45.0%; p = 0.64) and positive sputum smear results (M. bovis TB = 63.6%, M. tuberculosis TB = 67.3%; p = 0.40). Among M. tuberculosis TB patients, proportions categorized as high bacillary burden (36.2%) and medium bacillary burden (37.1%) differed significantly from those for M. bovis TB patients (28.3% and 40.4%, respectively) (p = 0.03).
At 2 months of treatment, 71% of M. bovis TB patients and 65% of M. tuberculosis TB patients showed conversion of their sputum cultures to negative (p<0.01) (Figure 2). By the end of 3 months of treatment, 86% of M. bovis TB patients and 83% of M. tuberculosis TB patients showed conversion of their sputum cultures to negative (Figure 2). On the basis of Cox proportional hazards regression modeling, we found that M. bovis TB patients had a higher hazard of conversion of sputum cultures to negative (aHR 1.18, 95% CI 1.04–1.33) relative to M. tuberculosis TB patients, after controlling for treatment administration type, sex, and the composite indicator for bacillary burden (Table 2). Directly observed therapy (aHR 1.12, 95% CI 1.09–1.15, relative to self-administered therapy) and female sex (aHR 1.15, 95% CI 1.12–1.18) were also found to increase the hazards of sputum-culture conversion. A graded response to bacillary burden was observed (low, aHR 1.68, 95% CI 1.63–1.74; medium, aHR 1.32, 95% CI 1.29–1.36) relative to high bacillary burden.
For patients given a standard 4-drug regimen in the United States, we found that the hazard of culture conversion over the first 3 months of anti-TB treatment was higher for patients with M. bovis TB than for patients with M. tuberculosis TB after controlling for treatment administration type, sex, and a composite indicator of bacillary burden. This finding was not documented previously and is especially intriguing because M. bovis is inherently resistant to pyrazinamide, a first-line anti-TB drug used during the first 2 months of treatment and credited with reducing relapse rates of the 6-month regimen to levels similar to the 9-month regimen without this drug (17). The implications of this inherent resistance are unknown. A systematic review of treatment for M. bovis TB concluded that the effect of 6-month versus 9-month treatment durations could not be determined because of a paucity of observational data (21). Our findings suggest that TB caused by M. bovis might not require a 9-month treatment regimen. However, this suggestion requires additional investigations.
We found that M. bovis TB patients had pulmonary and extrapulmonary involvement at diagnosis more frequently than M. tuberculosis TB patients, which is consistent with previous reports describing the transmission and epidemiology of M. bovis (2,3,22,23). However, our analysis showed that M. bovis TB patients with pulmonary disease might have been less infectious (i.e., had a lower bacillary burden) than M. tuberculosis TB patients. We also found similar proportions of cavitary lesions on chest imaging, which might indicate a similar pathogenicity between organisms when pulmonary disease is present. However, this finding could not be directly studied because NTSS does not collect data on smear grade. Alternatively, differential gene expression, proinflammatory macrophage response, growth in macrophages, and lipid profiles might have roles in explaining variability in bacillary populations between species (7–11). Further studies assessing differences in bacillary burden, and how anti-TB treatment is affected, will help clarify potential differences in treatment efficacy for M. tuberculosis complex species.
The true global burden of M. bovis TB is unknown and estimates are imprecise. Populations burdened by endemic zoonotic TB, such as large pastoralist populations who live near livestock and communities with increased consumption of unpasteurized dairy products, are often located where a specific M. bovis TB diagnosis is unlikely because extrapulmonary cases are not easily diagnosed and access to molecular technologies (e.g., genotyping) and drug susceptibility testing are not readily available (24–27). TB treatment strategies are moving toward shorter, more effective anti-TB regimens. If our finding of more rapid time to sputum-culture conversion for M. bovis TB could be replicated with more robust randomized clinical trial studies or hollow-fiber models (25,26), recommendations for treatment might be improved. Modifying these standards could reduce the recommended treatment length for potentially hundreds of thousands patients globally, assuming a conservative 2%–3% M. bovis TB prevalence estimate among the 9.6 million cases of TB reported each year (27). As research elucidates mycobacterial features and characteristics, individualized treatment regimens might be tailored to specific organisms. However, many potential new regimens include pyrazinamide as an essential drug throughout the entire treatment course (28).
This study and its findings are subject to the limitations of the national surveillance data we used. Historically, there has been variation among states in the reporting of HIV testing results (29). California, which reports more than half of all M. bovis TB cases nationally each year, began reporting HIV test results to the Centers for Disease Control and Prevention in 2011. This reporting limited our ability to analyze any association between HIV and time to sputum-culture conversion for this study. Although there is a standard recommendation for follow-up sputum collection frequency after initiation of TB treatment, clinician and patient variability in implementation is likely. However, because there is no reason to believe that this variation would be implemented differently for M. bovis TB patients versus M. tuberculosis TB patients, this limitation is not likely to have affected comparison on the basis of species.
We attempted to control for potential variations in treatment by including only patients initially given a standard 4-drug regimen (isoniazid, rifampin, ethambutol, and pyrazinamide daily for 2 months). However, some clinicians might have changed regimens after receiving genotyping results (e.g., M. bovis) or drug susceptibility testing results (e.g., pyrazinamide resistance). Thus, some patients with M. bovis genotyping results or pyrazinamide resistance might have received a different regimen at some point after the start of treatment. NTSS does not capture information on changes to regimens during the course of treatment or the date when drug susceptibility testing results were received, and we were unable to assess this information directly.
In 2013, time from treatment start date to date of linked genotyping results was a median of 107 days (range 15–365 days). Thus, for 50% of cases, clinicians would have received genotyping results after the event time of this analysis (i.e., 90 days), which would diminish any potential influence on our findings. Moreover, removing pyrazinamide from regimens used to treat M. bovis TB cases would have not have affected time to culture conversion; rather, it would have prolonged it.
Concurrent and immunosuppressive conditions, such as diabetes mellitus, end-stage renal disease, and hematologic or reticuloendothelial malignancies, which might influence time to culture conversion, were not routinely collected during our study period. These variables were included as part of routine reporting in 2009 and might be helpful in future studies (29). Because the proportion of race/ethnicity differed by bacterial species, and some race/ethnicities have a higher prevalence of concurrent conditions (e.g., diabetes mellitus) that might influence time to culture conversion, we attempted to run a separate hazard model on the basis of race/ethnicity, but this variable did not satisfy the proportional hazards assumption.
When we restricted analysis to only Hispanic persons, M. bovis TB patients had similar hazards of converting sputum cultures to negative (HR 1.20, 95% CI 1.10–1.35) as in our adjusted model (Table 2). This finding suggests that self-identifying as Hispanic had no effect on our main finding that M. bovis TB cases had a higher hazards of converting sputum cultures to negative relative to M. tuberculosis TB cases. Although we attempted to control for bacillary burden by using a novel composite variable, smear grade is not reported in NTSS, and our estimates on the effect of bacillary burden might be inaccurate.
Our findings should be interpreted with caution. Although we found earlier culture conversion for M. bovis TB patients than for M. tuberculosis TB patients, a larger proportion of M. bovis TB patients (8.9% vs. 5.1%) died during treatment. This finding suggests that earlier culture conversion does not necessarily lead to better clinical outcomes. Further laboratory studies should be conducted to better monitor and assess the time to sputum-culture conversion and clinical outcomes between these 2 M. tuberculosis complex species. If similar results are observed, further randomized clinical trials for treatment duration (and possibly treatment regimen) might be warranted.
Dr. Scott is a behavioral epidemiologist in the Strategic Information and Workforce Development Branch, Division of Global Immunization, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA. Her research interests are serving underserved populations globally through technical assistance, public health interventions, and recommendations based on interpretation of valid epidemiologic data and analyses.
Acknowledgments
We thank Steve Kammerer for providing technical expertise, assistance with SAS software, and revisions of figures.
This study was supported by the Centers for Disease Control and Prevention.
References
- Behr MA. Evolution of Mycobacterium tuberculosis. Adv Exp Med Biol. 2013;783:81–91. DOIPubMedGoogle Scholar
- Hlavsa MC, Moonan PK, Cowan LS, Navin TR, Kammerer JS, Morlock GP, et al. Human tuberculosis due to Mycobacterium bovis in the United States, 1995-2005. Clin Infect Dis. 2008;47:168–75. DOIPubMedGoogle Scholar
- Scott C, Cavanaugh JS, Pratt R, Silk BJ, LoBue P, Moonan PK. Human tuberculosis caused by Mycobacterium bovis in the United States, 2006–2013. Clin Infect Dis. 2016;63:594–601. DOIPubMedGoogle Scholar
- Majoor CJ, Magis-Escurra C, van Ingen J, Boeree MJ, van Soolingen D. Epidemiology of Mycobacterium bovis disease in humans, The Netherlands, 1993-2007. Emerg Infect Dis. 2011;17:457–63. DOIPubMedGoogle Scholar
- Rodwell TC, Moore M, Moser KS, Brodine SK, Strathdee SA. Tuberculosis from Mycobacterium bovis in binational communities, United States. Emerg Infect Dis. 2008;14:909–16. DOIPubMedGoogle Scholar
- Nicol MP, Wilkinson RJ. The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg. 2008;102:955–65. DOIPubMedGoogle Scholar
- Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328–37. DOIPubMedGoogle Scholar
- Krishnan N, Malaga W, Constant P, Caws M, Tran TH, Salmons J, et al. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS One. 2011;6:e23870. DOIPubMedGoogle Scholar
- Sarkar R, Lenders L, Wilkinson KA, Wilkinson RJ, Nicol MP. Modern lineages of Mycobacterium tuberculosis exhibit lineage-specific patterns of growth and cytokine induction in human monocyte-derived macrophages. PLoS One. 2012;7:e43170. DOIPubMedGoogle Scholar
- Pasipanodya JG, Moonan PK, Vecino E, Miller TL, Fernandez M, Slocum P, et al. Allopatric tuberculosis host-pathogen relationships are associated with greater pulmonary impairment. Infect Genet Evol. 2013;16:433–40. DOIPubMedGoogle Scholar
- Peters JS, Calder B, Gonnelli G, Degroeve S, Rajaonarifara E, Mulder N, et al. Identification of quantitative proteomic differences between Mycobacterium tuberculosis lineages with altered virulence. Front Microbiol. 2016;7:813. DOIPubMedGoogle Scholar
- Wallis RS, Doherty TM, Onyebujoh P, Vahedi M, Laang H, Olesen O, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2009;9:162–72. DOIPubMedGoogle Scholar
- Kurbatova EV, Gammino VM, Bayona J, Becerra MC, Danilovitz M, Falzon D, et al. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:1335–43. DOIPubMedGoogle Scholar
- Nahid P, Bliven EE, Kim EY, Mac Kenzie WR, Stout JE, Diem L, et al.; Tuberculosis Trials Consortium. Influence of M. tuberculosis lineage variability within a clinical trial for pulmonary tuberculosis. PLoS One. 2010;5:e10753. DOIPubMedGoogle Scholar
- Click ES, Winston CA, Oeltmann JE, Moonan PK, Mac Kenzie WR. Association between Mycobacterium tuberculosis lineage and time to sputum culture conversion. Int J Tuberc Lung Dis. 2013;17:878–84. DOIPubMedGoogle Scholar
- Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21.PubMedGoogle Scholar
- American Thoracic Society. CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1–77.
- Magdalena J, Supply P, Locht C. Specific differentiation between Mycobacterium bovis BCG and virulent strains of the Mycobacterium tuberculosis complex. J Clin Microbiol. 1998;36:2471–6.PubMedGoogle Scholar
- Ghosh S, Moonan PK, Cowan L, Grant J, Kammerer S, Navin TR. Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol. 2012;12:782–8. DOIPubMedGoogle Scholar
- Sparks FC. Hazards and complications of BCG immunotherapy. Med Clin North Am. 1976;60:499–509. DOIPubMedGoogle Scholar
- Lan Z, Bastos M, Menzies D. Treatment of human disease due to Mycobacterium bovis: a systematic review. Eur Respir J. 2016;48:1500–3. DOIPubMedGoogle Scholar
- LoBue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis. 2010;14:1075–8.PubMedGoogle Scholar
- Buss BF, Keyser-Metobo A, Rother J, Holtz L, Gall K, Jereb J, et al. Possible airborne person-to-person transmission of Mycobacterium bovis—Nebraska, 2014−2015. MMWR Morb Mortal Wkly Rep. 2016;65:197–201. DOIPubMedGoogle Scholar
- Fujiwara PI, Olea-Popelka F. Why it is important to distinguish Mycobacterium bovis as a causal agent of human tuberculosis. Clin Infect Dis. 2016;63:602–3. DOIPubMedGoogle Scholar
- Chilukuri D, McMaster O, Bergman K, Colangelo P, Snow K, Toerner JG. The hollow fiber system model in the non-clinical evaluation of antituberuclosis drug regimens. Clin Infect Dis. 2015;61(Suppl 1):S32–3. DOIPubMedGoogle Scholar
- Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis. 2015;61(Suppl 1):S18–24. DOIPubMedGoogle Scholar
- World Health Organization. Global tuberculosis report, 2016. Geneva: The Organization; 2016.
- Gualano G, Capone S, Matteelli A, Palmieri F. New antituberculosis drugs: from clinical trial to programmatic use. Infect Dis Rep. 2016;8:6569. DOIPubMedGoogle Scholar
- Yelk Woodruff RS, Pratt RH, Armstrong LR. The U.S. National Tuberculosis Surveillance System: a descriptive assessment of the completeness and consistency of data reported from 2008 to 2012. JMIR Public Health Surveill. 2015;1:e15. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 23, Number 3—March 2017
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Colleen Scott, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop A04, Atlanta, GA 30329-4017, USA
Top