Volume 23, Number 7—July 2017
Research
MERS-CoV Antibody Responses 1 Year after Symptom Onset, South Korea, 2015
Figure
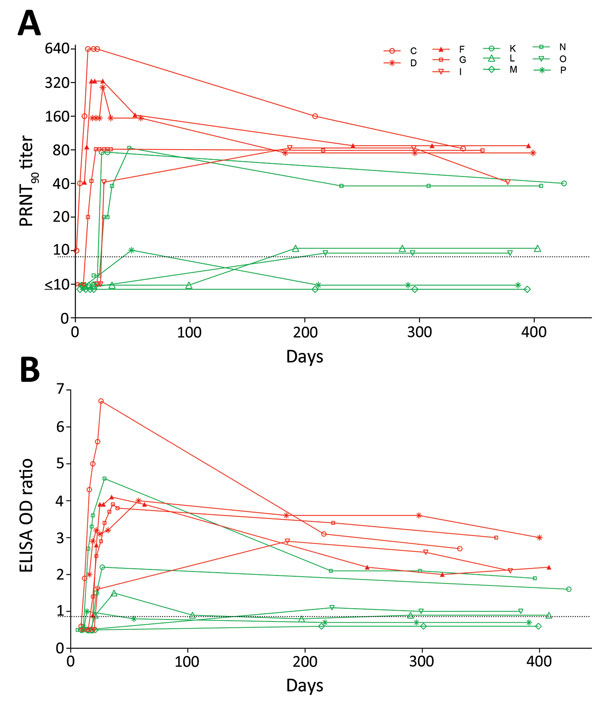
Figure. Middle East respiratory syndrome (MERS) coronavirus antibody titers in serially collected serum samples from 11 patients with reverse transcription PCR–confirmed symptomatic MERS, South Korea, 2015. PRNT90 titers (A) and MERS spike protein (S1) ELISA OD ratios (B) were determined at multiple time points 0 to >400 days after disease onset. The limit of detection was 10 for the PRNT, and the cutoff between negative and borderline samples for the S1 ELISA was an OD ratio of 0.8. Letters in key indicate patients; red indicates those with severe disease, and green indicates those with nonsevere disease. OD, optical density; PRNT90, >90% plaque-reduction neutralization test.
1These authors contributed equally to this article.
Page created: June 19, 2017
Page updated: June 19, 2017
Page reviewed: June 19, 2017
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.