Volume 23, Number 9—September 2017
Dispatch
Conveyance Contact Investigation for Imported Middle East Respiratory Syndrome Cases, United States, May 2014
Cite This Article
Citation for Media
Abstract
In 2014, the Centers for Disease Control and Prevention conducted conveyance contact investigations for 2 Middle East respiratory syndrome cases imported into the United States, comprising all passengers and crew on 4 international and domestic flights and 1 bus. Of 655 contacts, 78% were interviewed; 33% had serologic testing. No secondary cases were identified.
Two cases of imported Middle East respiratory syndrome (MERS) in the United States were confirmed in May 2014 in travelers from Saudi Arabia (1). Both persons were symptomatic at the time of travel. The Centers for Disease Control and Prevention (CDC) conducted large-scale (entire plane) contact investigations for the affected flights and for an interstate bus.
The investigation had 3 objectives: 1) notify travelers, 2) identify symptomatic contacts and facilitate prompt evaluation and isolation, and 3) determine the extent of onboard transmission. CDC approved the protocol as nonresearch.
We obtained passenger information and distributed it to state health departments as described (2). Foreign public health authorities were notified for US citizens abroad and foreign passport holders located outside the United States within the 14-day incubation period.
State health departments, CDC, or airline occupational health staff interviewed contacts using a standard questionnaire. Contacts interviewed within 14 days after exposure were advised to monitor themselves for fever and respiratory symptoms and to report symptoms to their state or local health department. Symptomatic contacts were reinterviewed about coexisting conditions and other exposure risks. When clinically indicated, state health departments coordinated specimen collection for testing by real-time reverse transcription PCR (rRT-PCR) (Table 1).
With contacts’ consent, serum specimens were collected at least 14 days after exposure. Serologic tests were a recombinant MERS coronavirus (MERS-CoV) nucleocapsid protein–based ELISA, followed by confirmatory testing for MERS-CoV–specific antibodies by immunofluorescence assay and microneutralization test on ELISA-positive serum specimens. Serologic assays were developed and performed at CDC, and microneutralization testing was done in a BioSafety Level 3 laboratory at CDC. Symptomatic contacts (contact definition category 1) were tested at state health department laboratories using the CDC MERS-CoV rRT-PCR (4).
Index case-patient 1 was a 65-year-old resident of Saudi Arabia who developed myalgia, fatigue, and a low-grade fever around April 18. On April 24, he flew from Riyadh to London, UK (Boeing 747–400, flight time 6 h 50 min), then London to Chicago, IL, USA (Boeing 767–300, 8 h 45 min). He then traveled to Indiana by bus (1 h 10 min). On April 27, cough, shortness of breath, increasing fever, and rhinorrhea developed; he was hospitalized April 28. MERS was diagnosed May 1 by the Indiana State Department of Health and confirmed May 2 by CDC (1).
Index case-patient 2, unconnected to case-patient 1, was a 43-year-old resident of Saudi Arabia who traveled on 2 international and 2 domestic US flights on May 1. He felt ill on his Riyadh–London flight (Boeing 777–300ER, 6 h 30 min); fever, chills, and myalgia developed on a flight from London to Boston, MA, USA (Boeing 767–400, 7 h 40 min) and cough on the domestic flights: Boston–Atlanta, GA, USA (McDonnell Douglas D-90, 2 h 50 min) and Atlanta–Orlando, FL, USA (Boeing 757, 1 h 30 min). On May 9, he sought care at a Florida emergency department with fever, cough, chills, and myalgia. Bilateral crackles were noted on exam; chest radiograph was normal. On May 11, the Florida Department of Health diagnosed MERS, subsequently confirmed by CDC (1).
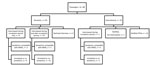
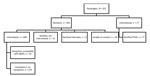
CDC investigated the 2 international flights inbound to the United States and 2 domestic flights. Nine additional US contacts were provided for the Riyadh–London flight of case-patient 1 and none for case-patient 2. CDC identified a total of 655 contacts for both persons. For case-patient 1, these were 89 passengers (Figure 1) and 12 crew members. The bus company reported 10 potential contacts but was able to identify only the driver and 5 passengers who had paid by credit card. For case-patient 2, a total of 521 passengers and 23 crew members were identified for the flights investigated by CDC (Figure 2).
For case-patient 1, passenger-locating information was provided to 17 health departments (1–12/state) the day MERS was confirmed, 8 days after exposure; interviews were sought during and after the incubation period. CDC notified 1 country for 22 passengers. For case-patient 2, contact information provided to 35 health departments (1–80/state), 11–12 days after the flight; 1 interview was sought. CDC notified 15 countries for 77 passengers.
Of the total 655 contacts, 631 (96%) were notified, of whom 512 (81%) were interviewed an average of 2.8 days after MERS confirmation. Of these, 435 (85%) reported no symptoms, 42 (8%) MERS-compatible symptoms, and 35 (7%) unrelated symptoms (Table 2).
Of 42 contacts with MERS-compatible symptoms, 7 reported acute respiratory illness with fever/feverishness, 32 acute respiratory illness without fever, 4 myalgia, 4 gastrointestinal symptoms, and 2 malaise. For 12 (29%), rRT-PCR was performed; MERS-CoV RNA was not detected. One contact who tested negative had been in the Middle East during the 14 days before the flight but reported no exposures of concern.
Blood was drawn for serum testing a mean of 33 days after exposure (median 21 days, range 9–90 days) from 218 (62%) aircraft passengers; 11 had unknown collection dates. Twelve (5%) specimens were collected within 14 days, 11 on day 12 or 13; the remainder were collected 14–90 days after the flight. Serologic test results were available for 11 (25%) passengers from both flights who sat within 2 rows of the case-patient and for 13 passengers who reported MERS-compatible symptoms. All serum tested negative for antibodies to MERS-CoV.
All flight crew were interviewed and reported no or unrelated symptoms. The bus driver and 4 of 5 passengers were interviewed and remained asymptomatic. No flight crew or bus contacts provided serum.
Close collaboration between state and local health departments, CDC, airline and bus industries, and federal partners was critical to rapidly complete these resource-intensive investigations. The 3 protocol objectives were met: achieving a high rate of timely notifications, identifying and evaluating symptomatic contacts, and using serology to detect transmission. The investigation detected no transmission on any of the conveyances. Concurrent household and healthcare facility contact investigations for these cases also did not identify MERS-CoV transmission (5).
At least 8 other aircraft and 2 bus investigations have been reported, totaling >400 evaluated contacts (6–13). Index case-patient symptoms have varied; flight times ranged from 1.5 to 10 hours. Contact definitions ranged from 2 adjacent seats in all directions to the entire plane; most common was within 2 rows of the index case-patient. Several investigations included laboratory testing of symptomatic or asymptomatic contacts. No transmission on aircraft or buses has been documented.
Limitations of our investigation included incomplete follow-up, self-reported symptoms, and potential recall bias. Cases may have been missed because a small number of travelers were interviewed or had specimens collected only during the 14-day incubation period.
The results of this and other investigations suggest the risk for MERS-CoV transmission on conveyances is low. Recent publications concluded that aircraft contact tracing required extensive preparation, resources, and passenger compliance; was an inconvenience to passengers; had mixed outcomes (14); and caused psychological distress to contacts (12). Our investigation required substantial resources of airlines, a bus company, local and state health departments, federal agencies, and foreign public health authorities. For future aircraft contact investigations for MERS, CDC will include only passengers seated within 2 rows of the index case-patient, although modifications may be made depending on circumstances. Comprehensive conveyance contact investigations with laboratory evaluation can guide future public health practice for emerging communicable diseases.
Dr. Lippold is CDC’s Occupational Health Clinic Medical Director. Her primary research interests include program evaluation, health equity, and healthcare quality.
Acknowledgment
For contact investigation work with passengers and crew and collaboration with CDC, we thank the state and territory public health agencies in Alabama, Arkansas, Arizona, California, Colorado, Connecticut, the District of Columbia, Florida, Georgia, Illinois, Indiana, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, New Hampshire, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, US Virgin Islands, Washington, and Wisconsin. We also thank our colleagues in CDC’s Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, and Division of State and Local Readiness and Division of Emergency Operations, Office of Public Health Preparedness and Response. We especially thank the many staff members who augmented our team at the Emergency Operations Center during this response and all of our CDC colleagues at CDC Quarantine Stations throughout the United States who worked tirelessly during this response.
References
- Bialek SR, Allen D, Alvarado-Ramy F, Arthur R, Balajee A, Bell D, et al.; Centers for Disease Control and Prevention (CDC). First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities - May 2014. MMWR Morb Mortal Wkly Rep. 2014/63;63:431–6.PubMedGoogle Scholar
- Regan JJ, Jungerman MR, Lippold SA, Washburn F, Roland E, Objio T, et al. Tracing airline travelers for a public health investigation: Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, 2014. Public Health Rep. 2016;131:552–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Interim patient under investigation (PUI) guidance and case definitions [cited 2017 May 9]. https://www.cdc.gov/coronavirus/mers/case-def.html
- Centers for Disease Control and Prevention. CDC laboratory testing for Middle East respiratory syndrome coronavirus (MERS-CoV) [cited 2017 May 8]. http://www.cdc.gov/coronavirus/mers/lab/lab-testing.html
- Breakwell L, Pringle K, Chea N, Allen D, Allen S, Richards S, et al. Lack of transmission among close contacts of patient with case of Middle East respiratory syndrome imported into the United States, 2014. Emerg Infect Dis. 2015;21:1128–34. DOIPubMedGoogle Scholar
- Tsiodras S, Baka A, Mentis A, Iliopoulos D, Dedoukou X, Papamavrou G, et al. A case of imported Middle East Respiratory Syndrome coronavirus infection and public health response, Greece, April 2014. Euro Surveill. 2014;19:20782. DOIPubMedGoogle Scholar
- Puzelli S, Azzi A, Santini MG, Di Martino A, Facchini M, Castrucci MR, et al. Investigation of an imported case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Florence, Italy, May to June 2013. Euro Surveill. 2013;18:20564. DOIPubMedGoogle Scholar
- Premila Devi J, Noraini W, Norhayati R, Chee Kheong C, Badrul AS, Zainah S, et al. Laboratory-confirmed case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Malaysia: preparedness and response, April 2014. Euro Surveill. 2014;19:20797. DOIPubMedGoogle Scholar
- Wu J, Yi L, Zou L, Zhong H, Liang L, Song T, et al. Imported case of MERS-CoV infection identified in China, May 2015: detection and lesson learned. Euro Surveill. 2015;20:21158. DOIPubMedGoogle Scholar
- Racelis S, de los Reyes VC, Sucaldito MN, Deveraturda I, Roca JB, Tayag E. Contact tracing the first Middle East respiratory syndrome case in the Philippines, February 2015. Western Pac Surveill Response J. 2015;6:3–7. DOIPubMedGoogle Scholar
- Mollers M, Jonges M, Pas SD, van der Eijk AA, Dirksen K, Jansen C, et al.; MERS-CoV Outbreak Investigation Team of the Netherlands. Follow-up of contacts of Middle East respiratory syndrome coronavirus–infected returning travelers, the Netherlands, 2014. Emerg Infect Dis. 2015;21:1667–9. DOIPubMedGoogle Scholar
- Parry-Ford F, Boddington N, Pebody R, Phin N, on behalf of the Incident Managemen C; Incident Management Team. Public health response to two incidents of confirmed MERS-CoV cases travelling on flights through London Heathrow Airport in 2014 – lessons learnt. Euro Surveill. 2015;20:21114. DOIPubMedGoogle Scholar
- Kang M, Song T, Zhong H, Hou J, Wang J, Li J, et al. Contact tracing for imported case of Middle East respiratory syndrome, China, 2015. Emerg Infect Dis. 2016;22:1644–6. DOIPubMedGoogle Scholar
- Huizer YL, Swaan CM, Leitmeyer KC, Timen A. Usefulness and applicability of infectious disease control measures in air travel: a review. Travel Med Infect Dis. 2015;13:19–30. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 23, Number 9—September 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Susan A. Lippold, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop A29, Atlanta, GA 30329-4027, USA
Top