Volume 24, Number 12—December 2018
Dispatch
Highly Pathogenic Avian Influenza A(H5N6) in Domestic Cats, South Korea
Figure 2
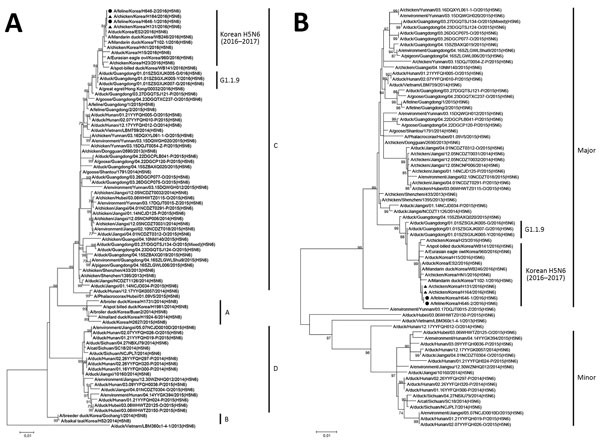
Figure 2. Maximum-likelihood phylogenetic tree of the hemagglutinin (A) and neuraminidase (B) gene segments for highly pathogenic avian influenza A(H5N6) viruses from cats, South Korea, and comparison viruses. Black circles indicate isolates from cats and triangles indicate chicken isolates from this study. Virus sequences from the GISAID EpiFlu database (http://platform.gisaid.org) and GenBank were used for each phylogenetic comparison. The genetic subclades are annotated to the right of the tree. The genetic clusters major, minor, and G1.1.9 were designated according to the criteria of Bi et al. (2). The number at each branch indicates a bootstrap value. Scale bars indicate nucleotide substitutions per site.
References
- Qi X, Cui L, Yu H, Ge Y, Tang F. Whole-genome sequence of a reassortant H5N6 avian influenza virus isolated from a live poultry market in China, 2013. Genome Announc. 2014;2:e00706–14. DOIPubMedGoogle Scholar
- Bi Y, Chen Q, Wang Q, Chen J, Jin T, Wong G, et al. Genesis, evolution and prevalence of H5N6 avian influenza viruses in China. Cell Host Microbe. 2016;20:810–21. DOIPubMedGoogle Scholar
- Yu Z, Gao X, Wang T, Li Y, Li Y, Xu Y, et al. Fatal H5N6 avian influenza virus infection in a domestic cat and wild birds in China. Sci Rep. 2015;5:10704. DOIPubMedGoogle Scholar
- Li X, Fu Y, Yang J, Guo J, He J, Guo J, et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect Genet Evol. 2015;36:462–6. DOIPubMedGoogle Scholar
- Lee EK, Song BM, Lee YN, Heo GB, Bae YC, Joh SJ, et al. Multiple novel H5N6 highly pathogenic avian influenza viruses, South Korea, 2016. Infect Genet Evol. 2017;51:21–3. DOIPubMedGoogle Scholar
- Son K, Kim YK, Oem JK, Jheong WH, Sleeman JM, Jeong J. Experimental infection of highly pathogenic avian influenza viruses, Clade 2.3.4.4 H5N6 and H5N8, in Mandarin ducks from South Korea. Transbound Emerg Dis. 2018;65:899–903. DOIPubMedGoogle Scholar
- Lyoo KS, Na W, Phan LV, Yoon SW, Yeom M, Song D, et al. Experimental infection of clade 1.1.2 (H5N1), clade 2.3.2.1c (H5N1) and clade 2.3.4.4 (H5N6) highly pathogenic avian influenza viruses in dogs. Transbound Emerg Dis. 2017;64:1669–75. DOIPubMedGoogle Scholar
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, et al. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006;12:681–3. DOIPubMedGoogle Scholar
- Swayne DE, Alexander DJ. Confirmation of nephrotropism and nephropathogenicity of three low-pathogenic chicken-origin influenza viruses for chickens. Avian Pathol. 1994;23:345–52. DOIPubMedGoogle Scholar
- Bonfante F, Fusaro A, Zanardello C, Patrono LV, De Nardi R, Maniero S, et al. Lethal nephrotropism of an H10N1 avian influenza virus stands out as an atypical pathotype. Vet Microbiol. 2014;173:189–200. DOIPubMedGoogle Scholar
- Cardona CJ, Xing Z, Sandrock CE, Davis CE. Avian influenza in birds and mammals. Comp Immunol Microbiol Infect Dis. 2009;32:255–73. DOIPubMedGoogle Scholar
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. DOIPubMedGoogle Scholar
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–6. DOIPubMedGoogle Scholar
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RA, Amonsin A, Payungporn S, et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004;10:2189–91. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.