Volume 24, Number 6—June 2018
Research Letter
Molecular Diagnosis of Taenia saginata Tapeworm Infection in 2 Schoolchildren, Myanmar
Abstract
Taenia saginata is the most common human tapeworm worldwide but has been unknown in Myanmar. In 2017, fecal examination in Yangon, Myanmar, revealed eggs of Taenia species in 2 children from a monastic school. Several proglottids expelled after medication with praziquantel were morphologically and molecularly confirmed to be T. saginata tapeworms.
Human taeniasis is a parasitic infection caused by tapeworm species including Taenia saginata, T. solium, and T. asiatica (1). T. saginata tapeworm infection is acquired through ingestion of raw or undercooked beef; pork is the infection source for T. solium and T. asiatica tapeworms (1). Because of differences in the life cycle, geographic distribution of these parasites can be affected by regional lifestyle, including dietary habit. Little is known about taeniasis in Myanmar. We report 2 cases of taeniasis caused by T. saginata tapeworms in Myanmar.
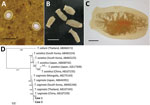
In June 2017, the Korea Association of Health Promotion, in cooperation with the National Health Laboratory, Myanmar, conducted a survey of intestinal parasitic infections near the Yangon region of Myanmar. The Institutional Review Board of the Ministry of Health and Sports, Myanmar (Ethical Review Committee no. 005117) approved the study. A total of 467 fecal samples were obtained from school-age children living in the district of Shwe Pyi Thar, Myanmar. In fecal examination using the Kato-Katz thick-smear technique, we found the eggs of Taenia tapeworms in 2 brothers, 8 and 10 years of age (Figure, panel A). They had never traveled out of Myanmar, and there was no possibility of consumption of imported beef. The younger boy had no specific gastrointestinal symptoms, but actively moving tapeworm segments had been found in his feces a year earlier. The older boy also had no special gastrointestinal symptoms.
The children were orphans who lived in Shan State and moved to a monastic school in the Shwe Pyi Thar area in 2015. They relied on traditional food donated from villagers, but food history related to raw beef or pork with their origin (imported or not) was unclear. However, the children reported that the Shan population usually enjoys traditional cuisine, fermented with rice and raw beef or pork.
A sample of whole feces was collected from each child 1 day after treatment with praziquantel (10 mg/kg single oral dose). Seven tapeworm segments were recovered in feces (Figure, panel B), and >13 uterine segments filled with eggs were observed (Figure, panel C). We extracted genomic DNA from segments by using the DNeasy Blood &Tissue Kit (QIAGEN, Hilden, Germany) as recommended by the manufacturer. The mitochondrial cytochrome c oxidase 1 (cox1) gene was targeted in PCR amplification and sequencing. The PCR amplification was performed with primers T1F (5′-ATA TTT ACT TTA GAT CAT AAG CGG-3′) and T1R (5′-ACG AGA AAA TAT ATT AGT CAT AAA-3′), and conditions according to a previous study (2). Sequencing of the 502 bp cox1 gene showed 98.8%–99.6% nt identity with T. saginata, but 93.8%–94.4% with T. asiatica, and 87.8%–88.0% with T. solium. These specimens were molecularly close to T. saginata tapeworms reported from various Asian countries but far from T. asiatica or T. solium tapeworms (Figure, panel D). Our results demonstrate that T. saginata tapeworms caused the taeniasis in these 2 children.
T. saginata tapeworms have a global distribution and are known to be endemic to Southeast Asia. Recently, epidemiologic studies of taeniasis in Myanmar have been performed; however, they focused on T. solium cysticercosis in pigs in Nay Pyi Taw area and seropositivity of refugee camp residents on the Thailand–Myanmar border (3,4). A report of T. saginata in Myanmar described only an experimental infection in animals, not human infections (5). Fecal examination might not be helpful in cases of taeniasis because Taenia spp. eggs are not differentiated morphologically. Instead, taeniasis can be diagnosed through the morphology of gravid proglottids or by immunologic or molecular techniques. In the cases we reported, a history of active movement of proglottids and >13 uterine branches in recovered segments indicated T. saginata rather than T. solium tapeworm infection, but T. asiatica tapeworms could not be fully ruled out. Although T. asiatica tapeworms can differ morphologically from T. saginata tapeworms (6), the distinctions could not always be found in each strobila; thus, molecular analyses were required to clearly distinguish them (2,7). We analyzed mitochondrial cox1 of the Taenia tapeworm specimens and showed that the sequences clustered with T. saginata tapeworms reported from several Asia countries, but far from those of T. asiatica and T. solium tapeworms. Recently, human infections caused by hybrid infection with T. saginata and T. asiatica tapeworms in Laos were determined by sequencing the DNA polymerase delta region (8). Thus, for further studies, it may be useful to analyze not only the mitochondrial gene but also nuclear DNA.
Although epidemiologic surveys of T. saginata tapeworms have not been conducted in Myanmar, there is a strong possibility of the domestic occurrence of human taeniasis from consumption of undercooked beef or pork. Our report suggests that surveys of the prevalence and associated factors of human taeniases are urgently needed in Myanmar.
Dr. Won is an assistant professor at Chonnam National University Medical School, Gwangju, South Korea. Her primary research interest is clinical parasitology, particularly in terms of host–parasite interactions.
Acknowledgment
This work was partly supported by the Institute of Parasitic Diseases, Korea Association of Health Promotion and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, South Korea (NRF—2016R1C1B1009746).
References
- Chai JY. Human taeniasis in the Republic of Korea: hidden or gone? Korean J Parasitol. 2013;51:9–17. DOIPubMedGoogle Scholar
- Cho J, Jung BK, Lim H, Kim MJ, Yooyen T, Lee D, et al. Four cases of Taenia saginata infection with an analysis of COX1 gene. Korean J Parasitol. 2014;52:79–83. DOIPubMedGoogle Scholar
- Khaing TA, Bawm S, Wai SS, Htut Y, Htun LL. Epidemiological survey on porcine cysticercosis in Nay Pyi Taw area, Myanmar. J Vet Med. 2015;2015:340828. DOIPubMedGoogle Scholar
- McCleery EJ, Patchanee P, Pongsopawijit P, Chailangkarn S, Tiwananthagorn S, Jongchansittoe P, et al. Taeniasis among refugees living on Thailand–Myanmar border, 2012. Emerg Infect Dis. 2015;21:1824–6. DOIPubMedGoogle Scholar
- Fan PC, Lin CY, Chen LM. Experimental infection and morphology of Taenia saginata (Burma strain) in domestic animals. Ann Trop Med Parasitol. 1992;86:317–8. DOIPubMedGoogle Scholar
- Eom KS, Rim HJ. Morphologic descriptions of Taenia asiatica sp. n. Korean J Parasitol. 1993;31:1–6. DOIPubMedGoogle Scholar
- Sato MO, Sato M, Yanagida T, Waikagul J, Pongvongsa T, Sako Y, et al. Taenia solium, Taenia saginata, Taenia asiatica, their hybrids and other helminthic infections occurring in a neglected tropical diseases’ highly endemic area in Lao PDR. PLoS Negl Trop Dis. 2018;12:e0006260. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: May 07, 2018
Table of Contents – Volume 24, Number 6—June 2018
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jong-Yil Chai, Institute of Parasitic Diseases, Korea Association of Health Promotion, Seoul 07649, South Korea
Top