Volume 25, Number 1—January 2019
Research Letter
Trends in Azole Resistance in Aspergillus fumigatus, the Netherlands, 1994–2016
Abstract
We investigated azole resistance in Aspergillus fumigatus in a tertiary reference hospital in the Netherlands during 1994–2016. The 5-year patient-adjusted proportion of resistance increased from 0.79% for 1996–2001 to 4.25% for 2002–2006, 7.17% for 2007–2011, and 7.04% for 2012–2016. However, we observed substantial variation between years.
Azole resistance is increasingly reported in Aspergillus fumigatus and is now found all around the world (1). Most studies have investigated the presence of resistance in environmental or clinical samples over a limited time, but longitudinal resistance studies are lacking. It is important to determine if azole resistance frequency shows increasing trends over time and if the distribution of resistance mutations changes over time. We have previously reported the emergence of azole resistance from 1994–2007 for the Radboud University Medical Center (RUMC) in Nijmegen, the Netherlands (2). Here, we describe trends in resistance frequency and distributions of mutations over a 23-year period, from 1994 through 2016.
All clinical A. fumigatus isolates cultured at RUMC are screened for azole resistance. Before 2009, isolates were screened using an agar-slant supplemented with 4 mg/L itraconazole (2). During 2009–2011 a 4-well plate was developed containing itraconazole (4 mg/L), voriconazole (1 mg/L), posaconazole (0.5 mg/L), and a growth control. Since 2012, we have used a commercial agar-based screening system (VIPcheck, http://www.vipcheck.nl) containing 2 mg/L of voriconazole (3,4). We performed EUCAST susceptibility testing (http://www.eucast.org) and sequencing of the cyp51A gene and promoter on isolates that grew on azole-containing wells (2).
We calculated the resistance frequency of azole-resistant A. fumigatus isolates for each year, using the number of cultured isolates as denominator. Furthermore, we calculated the patient-adjusted proportion of resistance for each year and for each 5-year period, using the number of patients with a resistant isolate as numerator and of culture-positive patients as denominator. We determined by χ2 test whether trends of resistance frequency were statistically significant (p<0.05). When 2 different resistance mechanisms were recovered from a single patient, we counted the patient once for determining the resistance proportion but used both isolates to determine the resistance frequency.
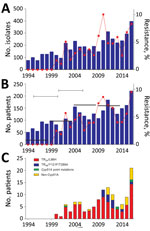
Over the 23-year period, 4,268 A. fumigatus isolates were cultured from 2,051 patients, a resistance frequency of 4.2% (179/4,268 isolates). Azole-resistant A. fumigatus was found in 109/2,051 (5.3%) culture-positive patients (Figure, panel A). The patient-adjusted resistance proportion increased from 0% in 1997 to 9.5% in 2016 (Figure, panel B). The increase of resistance was not statistically significant when the proportion of resistance for each consecutive year was analyzed. However, the 5-year proportion of resistance increased from 0.79% for 1996–2001 to 4.25% for 2002–2006, 7.17% for 2007–2011, and 7.04% for 2012–2016 (Figure, panel A). The increases in resistance for 2002–2006 compared with 1996–2001 and for 2007–2011 compared with 2002–2006 were statistically significant (p<0.05) (Figure, panel B).
TR34/L98H was the most prevalent resistance mutation over the 23-year period; it was present in 77/109 (70.6%) patients with drug-resistant A. fumigatus (Figure, panel C). TR46/Y121F/T289A was found in 2 patients in 2010, 1 in 2011, and 1 in 2012 but only twice during 2013–2016. In recent years, resistant phenotypes without Cyp51A mutations were encountered more frequently than phenotypes with the mutation (Figure, panel C).
We observed an increasing trend in azole resistance prevalence in clinical A. fumigatus isolates until 2011, using number of patients with a positive culture as denominator. After 2011, the 5-year proportion of resistance remained stable. The advantages and disadvantages of different approaches of reporting resistance frequency remain under debate (5). Experts have recommended 10% resistance rate as the threshold for reconsideration of primary antifungal therapy (6), which indicates a need for consensus in how to determine resistance rates (5).
Although we found a substantial increase in azole resistance frequency over time, our study showed variation between consecutive years. Therefore, analysis of culture-positive patients over multiple years is required to determine local resistance epidemiology. Furthermore, resistance rates calculated using Aspergillus disease as denominator provide more information to support changes in empiric treatment decisions, but the low number of culture-positive patients in risk groups makes it difficult to obtain accurate estimates.
Various factors might have caused bias over the long period. The method of resistance detection changed from an agar-slant containing itraconazole to a system that contained 3 azoles. Voriconazole- or posaconazole-resistant isolates with low itraconazole MICs may have been initially missed, but this phenotype is very uncommon (7). Other factors include increased awareness of resistance, the policy to screen multiple A. fumigatus colonies after observation of patients with mixed azole-susceptible and azole-resistant infection (8), and local changes in aspergillus disease risk groups.
Resistance was dominated by environmental resistance mutations TR34/L98H and TR46/Y121F/T289A, although the number of patients with TR46/Y121F/T289A decreased in recent years. Furthermore, in the last 5 years, ≈15% of resistant isolates harbored a wild-type Cyp51A gene, suggesting that other resistance mechanisms may be emerging. Because commercial PCR tests detect only resistance mechanisms with TR34 (9) or TR34 and TR46 (10), our observation is relevant for using these assays in culture-negative patients.
In summary, our study indicates an increasing azole resistance trend in clinical A. fumigatus isolates in the Netherlands. Furthermore, our results highlight difficulties encountered in establishing local epidemiology of this resistance.
Dr. Buil is currently a resident in clinical microbiology and a PhD student at Radboud University Medical Center in Nijmegen, the Netherlands. His research interests include the diagnosis of azole-resistant aspergillosis and preclinical studies of new antifungal agents.
Acknowledgments
This study was supported by internal funding.
Disclosures: P.E.V. has received research grants from Gilead Sciences, Astellas, Merck Sharp & Dohme (MSD), F2G, and BioRad, is a speaker for Gilead Sciences and MSD, and is on the advisory boards for Pfizer, MSD, and F2G. All other authors have nothing to declare.
References
- Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62:362–8. DOIPubMedGoogle Scholar
- Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:e219. DOIPubMedGoogle Scholar
- Buil JB, van der Lee HAL, Rijs AJMM, Zoll J, Hovestadt JAMF, Melchers WJG, et al. Single centre evaluation of an agar-based screening for azole resistance in Aspergillus fumigatus by using VIPcheck. Antimicrob Agents Chemother. 2017;61:e01250–17. DOIPubMedGoogle Scholar
- Arendrup MC, Verweij PE, Mouton JW, Lagrou K, Meletiadis J. Multicentre validation of 4-well azole agar plates as a screening method for detection of clinically relevant azole-resistant Aspergillus fumigatus. J Antimicrob Chemother. 2017;72:3325–33. DOIPubMedGoogle Scholar
- Verweij PE, Lestrade PP, Melchers WJ, Meis JF. Azole resistance surveillance in Aspergillus fumigatus: beneficial or biased? J Antimicrob Chemother. 2016;71:2079–82. DOIPubMedGoogle Scholar
- Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21-22:30–40. DOIPubMedGoogle Scholar
- van Ingen J, van der Lee HA, Rijs TA, Zoll J, Leenstra T, Melchers WJ, et al. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother. 2015;70:178–81. DOIPubMedGoogle Scholar
- Kolwijck E, van der Hoeven H, de Sévaux RG, ten Oever J, Rijstenberg LL, van der Lee HA, et al. voriconazole-susceptible and voriconazole-resistant Aspergillus fumigatus coinfection. Am J Respir Crit Care Med. 2016;193:927–9. DOIPubMedGoogle Scholar
- Chong GM, van der Beek MT, von dem Borne PA, Boelens J, Steel E, Kampinga GA, et al. PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bronchoalveolar lavage: a multicentre validation of the AsperGenius assay® in 201 patients with haematological disease suspected for invasive aspergillosis. J Antimicrob Chemother. 2016;71:3528–35. DOIPubMedGoogle Scholar
- Dannaoui E, Gabriel F, Gaboyard M, Lagardere G, Audebert L, Quesne G, et al. Molecular diagnosis of invasive aspergillosis and detection of azole resistance by a newly commercialized PCR kit. J Clin Microbiol. 2017;55:3210–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: December 04, 2018
Table of Contents – Volume 25, Number 1—January 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jochem B. Buil, Department of Medical Microbiology, Radboud University Medical Center, PO Box 9101, 6500 HB Nijmegen, the Netherlands
Top