Volume 25, Number 1—January 2019
Dispatch
Dengue Virus IgM Serotyping by ELISA with Recombinant Mutant Envelope Proteins
Figure 2
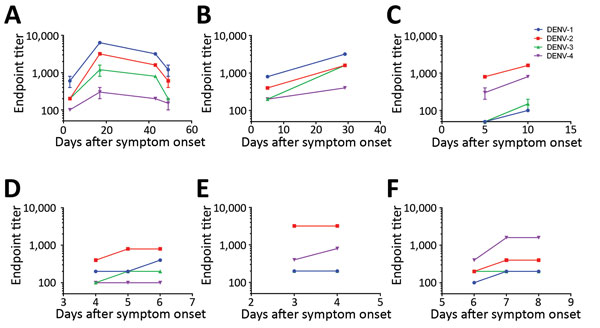
Figure 2. DENV IgM ELISA endpoint titers, by serotype, of paired patient serum samples acquired at 2–4 time points after symptom onset, 2013–2018. A–C) Serum samples from returning travelers with residence in Germany: DENV-1 positive (A, B); DENV-2 positive (C). D–F) Serum samples from residents of DENV-endemic country Sri Lanka: DENV-2 positive (D, E); DENV-4 positive (F). Data lines indicate average titers; error bars indicate SDs. The antigens in the ELISA were Equad proteins (i.e., envelope protein from each DENV serotype with 4 amino acid changes T76R, Q77E, W101R, and L107R). DENV, dengue virus.
Page created: December 18, 2018
Page updated: December 18, 2018
Page reviewed: December 18, 2018
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.