Volume 25, Number 11—November 2019
Research Letter
Mycobacterium microti Infection in Free-Ranging Wild Boar, Spain, 2017–2019
Abstract
Mycobacterium microti is a member of the Mycobacterium tuberculosis complex that causes pathology in many mammals. M. microti infections have been found in some countries in Europe. We report an outbreak of tuberculosis caused by M. microti in wild boars in Spain.
Mycobacterium microti is a member of the Mycobacterium tuberculosis complex (MTBC), which also includes M. tuberculosis and M. bovis, the main causes of human and animal tuberculosis (TB), respectively. Even though voles and other wild small rodents were initially identified as its natural hosts (1), M. microti can cause pathology in a wide range of mammals, including pets, livestock, wildlife (2–5), and humans (6). M. microti infections have been previously reported in several countries in Europe, including Switzerland, Italy, and France (3,7–9). We report an outbreak of tuberculosis caused by M. microti in free-ranging wild boars in the Iberian Peninsula in Spain.
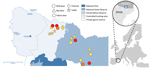
During June 2017–March 2019, a total of 9 free-ranging wild boars with lesions associated with TB were detected in the outbreak area, covering ≈3,000 hectares in the Catalan Pyrenees (Figure). TB was confirmed histologically, first by hematoxylin and eosin staining (9/9) and then by Ziehl-Neelsen staining of acid-fast bacilli (7/9). In all cases, submandibular lymph nodes showed granulomatous necrotizing lymphadenitis, sometimes with scant acid-fast bacilli, similar to that found in M. microti infections previously described in wild boar, which were generally confined to lymph nodes in the head (7,8).
To confirm the causative agent for these infections, we extracted DNA from tissue samples (DNAExtract-VK, Vacunek, http://vacunek.com) and performed real-time PCR (TBC-VK, Vacunek), which confirmed MTBC in 6 of 9 suspected cases. DVR spoligotyping at VISAVET Health Surveillance Centre, Universidad Complutense de Madrid, identified the pathogen in 4 of the 6 confirmed MTBC cases as M. microti (spoligopattern SB0423; Mycobacterium bovis spoligotype database, http://www.mbovis.org). In the other 2 cases, the laboratory was unable to determine the species because of low DNA load from the sample. This result likely was due to the slow in vitro growth rate of M. microti in infected animals, which makes it difficult to isolate in routine diagnostic laboratories.
Spoligopattern SB0423 is included in a phylogenetic cluster with spoligopattern SB0112, also associated with M. microti, on the basis of neighbor joining. Both spoligotypes are localized in the eastern French Pyrenees, close to the borders with Spain and Andorra (3). Most M. microti cases in France, found in cats, dogs, and llamas during 2005–2016 and, since 2015, in wild boars and badgers, have been found within 50 km of the outbreak area in Spain. The 2017 M. microti cases described in this report were found closer to the border with France; the remaining 2 cases, detected in 2019, were localized near the southern limit of the outbreak area (Figure).
In the outbreak area, up to 18 animals in 3 cattle herds showed positive results for a single intradermal tuberculin skin test (Figure). However, none of these animals showed gross lesions in target tissues (i.e., lungs, pulmonary, and retropharyngeal lymph nodes) at a slaughterhouse or positive results to mycobacterial culture and PCR. Similarly, in a recent case in France, a cow reacting to a TB skin test did not show TB-like lesions in respiratory tissues and returned negative results from cultures, but M. microti DNA was finally detected in retropharyngeal lymph nodes only by using advanced molecular techniques (5). These results indicate that cattle exposed to M. microti may induce positive results to diagnostic tests performed in bovine TB eradication campaigns. M. microti infection can sometimes cause visible lesions in cattle (2), but the fact that M. microti are natural knockouts for the virulence-related RD1mic genomic region (10) may indicate a lower pathogenicity compared with other MTBC species and account for these negative test results.
We tested additional wild ungulates in the outbreak area and found that 6 (2 red deer, 1 fallow deer, and 3 chamois) were seropositive for MTBC using a MPB83-specific IgG indirect ELISA test (Figure). Unfortunately, no tissue samples were submitted to examine for lesions or to detect and identify mycobacteria. However, overall positive results for M. microti and the absence of other MTBC strains during the 2017–2019 period in the outbreak area suggest a multihost circulation of M. microti. Because voles are known maintenance hosts of M. microti (1), further investigation of wild small rodent populations in the Outbreak area could determine the epidemiology of this outbreak in greater detail.
These findings, together with previously reported cases nearer the border between France and Spain, indicate a transboundary circulation of M. microti across the Pyrenean border that should be taken into account for wildlife TB surveillance. Coordinated action between animal health authorities and laboratories in Spain and France is required, as well as the improvement of livestock management and biosecurity practices.
Dr. Pérez de Val is a researcher at Institut de Recerca i Tecnologia Agroalimentàries–Centre de Recerca en Sanitat Animal, Bellaterra, Spain, where he conducts research on animal TB.
Acknowledgments
We thank the forest guard body of the Catalan Government and Vedat Pirineus S.L. for wildlife samples collection. We are also grateful to Maite Martín, Zoraida Cervera, Sierra Espinar, and Mónica Pérez for their outstanding technical assistance.
This work was funded by the Department of Agriculture, Livestock, Fisheries and Food (DARP) of the Government of Catalonia. IRTA is supported by Centres de Recerca de Catalunya (CERCA) Programme/Generalitat de Catalunya (www.cerca.cat).
References
- Cavanagh R, Begon M, Bennett M, Ergon T, Graham IM, De Haas PE, et al. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J Clin Microbiol. 2002;40:3281–5. DOIPubMedGoogle Scholar
- Smith NH, Crawshaw T, Parry J, Birtles RJ. Mycobacterium microti: More diverse than previously thought. J Clin Microbiol. 2009;47:2551–9. DOIPubMedGoogle Scholar
- Michelet L, de Cruz K, Karoui C, Hénault S, Boschiroli M. Mycobacterium microti, an unrecognized tubercular agent [in French]. Epidémiol Santé Anim. 2017;71:129–38.
- Michelet L, de Cruz K, Phalente Y, Karoui C, Hénault S, Beral M, et al. Mycobacterium microti infection in dairy goats, France. Emerg Infect Dis. 2016;22:569–70. DOIPubMedGoogle Scholar
- Michelet L, De Cruz K. Mycobacterium microti infection in a cow in France. Vet Rec. 2017;180:429.PubMedGoogle Scholar
- Panteix G, Gutierrez MC, Boschiroli ML, Rouviere M, Plaidy A, Pressac D, et al. Pulmonary tuberculosis due to Mycobacterium microti: a study of six recent cases in France. J Med Microbiol. 2010;59:984–9. DOIPubMedGoogle Scholar
- Schöning JM, Cerny N, Prohaska S, Wittenbrink MM, Smith NH, Bloemberg G, et al. Surveillance of bovine tuberculosis and risk estimation of a future reservoir formation in wildlife in Switzerland and Liechtenstein. PLoS One. 2013;8:
e54253 . DOIPubMedGoogle Scholar - Boniotti MB, Gaffuri A, Gelmetti D, Tagliabue S, Chiari M, Mangeli A, et al. Detection and molecular characterization of Mycobacterium microti isolates in wild boar from northern Italy. J Clin Microbiol. 2014;52:2834–43. DOIPubMedGoogle Scholar
- Chiari M, Ferrari N, Giardiello D, Avisani D, Pacciarini ML, Alborali L, et al. Spatiotemporal and ecological patterns of Mycobacterium microti infection in wild boar (Sus scrofa). Transbound Emerg Dis. 2016;63:e381–8. DOIPubMedGoogle Scholar
- Brodin P, Eiglmeier K, Marmiesse M, Billault A, Garnier T, Niemann S, et al. Bacterial artificial chromosome-based comparative genomic analysis identifies Mycobacterium microti as a natural ESAT-6 deletion mutant. Infect Immun. 2002;70:5568–78. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: October 03, 2019
Table of Contents – Volume 25, Number 11—November 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Bernat Pérez de Val, Institut de Recerca i Tecnologia Agroalimentàries–Centre de Recerca en Sanitat Animal, Edifici CReSA. Campus UAB (Bellaterra), Bellaterra, Barcelona 08193, Spain
Top