Volume 25, Number 4—April 2019
Research Letter
Rickettsia parkeri and Candidatus Rickettsia andeanae in Tick of the Amblyomma maculatum Group, Mexico
Cite This Article
Citation for Media
Abstract
We report Rickettsia parkeri and Candidatus Rickettsia andeanae in ticks of the Amblyomma maculatum group collected from dogs in Sonora, Mexico. Molecular characterization of these bacteria was accomplished by DNA amplification and sequence analysis of portions of the rickettsial genes gltA, htrA, ompA, and ompB.
Rickettsia parkeri, a member of the spotted fever group Rickettsia (SFGR), was initially identified in Amblyomma maculatum ticks in 1937, but not until 2004 was the first confirmed human infection reported (1). Through 2018, at least 9 cases of R. parkeri rickettsiosis have been identified across several mountainous locations in southern Arizona close to the US–Mexico border, and ticks of the A. maculatum group infected with R. parkeri have been collected from this region (2).
Epidemic levels of Rocky Mountain spotted fever (RMSF), a severe and frequently fatal spotted fever rickettsiosis, have been identified throughout regions of northern Mexico, including the state of Sonora (3). R. rickettsii (the agent of RMSF) is the only SFGR species linked with spotted fever rickettsiosis in Sonora; nonetheless, a unique lineage of R. parkeri associated with the Dermacentor parumapertus tick was reported recently from northern Mexico (4). Sonora shares an international border with Arizona; the recent identification of R. parkeri in Amblyomma ticks in southern Arizona (2) prompted our investigation during July 2016 for ticks in northern Sonora to determine whether another pathogenic SFGR species could also exist in Mexico.
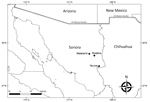
The study area comprised 3 localities in Sonora (Yecora, 28°22′16″N, 108°55′32″W; Matarachi, 28°73′54′′N, 108°95′87′′W; and Mulatos, 28°65′85′′N, 108°74′78′′W) (Figure), each with a humid subtropical climate situated at ≈1,500 m, with vegetation comprising predominantly oak forests and grasslands (Appendix Figure, panel A). During August 2016 and September 2017, we inspected free-roaming dogs (Canis lupus familiaris) for attached or crawling ticks (Appendix Figure, panel B). We identified ticks using morphological characteristics (5).
For ticks collected in 2016, we dissected a portion of the posterolateral idiosoma from each specimen and extracted DNA using a DNeasy Blood and Tissue Kit (QIAGEN, https://www.qiagen.com). We screened DNA extracts using a Rickettsia genus–specific real-time PCR with primers PanR8_F and PanR8_R, probe PanR8_P (6), and 5 μL of DNA template. Samples with cycle threshold values <40 were tested by a PCR targeting a segment of the ompA gene using primers Rr190.70p and 190–701 (7). We also tested 1 positive sample using a nested PCR to amplify a segment of the 17-kDa antigen gene with primers R17122 and R17500 (8) in the primary amplification and TZ15 and TZ16 (9) in the nested amplification. Specimens collected in 2017 were processed similarly; however, we screened DNA extracts using a conventional PCR to amplify a segment of the gltA gene (4). We subsequently tested positive samples by conventional PCRs targeting fragments of the ompA and ompB, genes using primers and conditions described elsewhere (4). PCR products were sequenced using an ABI 3500 genetic analyzer with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, https://www.thermofisher.com). Sequences were assembled using Geneious R10 (Biomatters Ltd., https://www.geneious.com) and compared with GenBank data using blastn analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
We collected a total of 31 A. maculatum group ticks from northern Sonora (14 specimens from Yecora in 2016 and 17 from Matarachi and Mulatos in 2017). Three ticks collected in 2017 were deposited as voucher specimens in the Colección del Laboratorio de Acarología, Universidad Nacional Autónoma de México (Mexico City, Mexico).
We amplified a sequence demonstrating complete identity to the homologous 590-bp segment of the ompA gene of R. parkeri strain Maculatum 20 (GenBank accession no. U43802) from 1 (7%) of 14 ticks collected in 2016. We further amplified sequences demonstrating complete identities to the homologous segments of the ompA (590 bp) and 17-kDa antigen (208 bp) genes of Candidatus Rickettsia andeanae (GenBank accession nos. KF179352 and KY402193, respectively) from another tick in this group and sequences revealing complete identity to each other and to the homologous segments of the gltA (760 bp), ompB (760 bp), and ompA (490 bp) genes of R. parkeri strain Portsmouth (GenBank accession no. CP003341.1) from 10 (71%) of 14 ticks evaluated from the 2017 collection.
We identified DNA of R. parkeri and Candidatus Rickettsia andeanae in A. maculatum group ticks in northern Sonora. R. parkeri causes a disease less severe than RMSF and should be suspected in patients with an eschar, rash, and lymphadenopathy (1). The results of this investigation suggest that R. parkeri could contribute to at least some of the cases of spotted fever rickettsiosis described in Sonora and possibly in other regions of Mexico where A. maculatum group ticks are found. Differentiation between these 2 diseases is important, principally because there are no reports of fatal disease caused by R. parkeri. Nonetheless, clinical suspicion of any SFGR requires immediate treatment with doxycycline. Candidatus Rickettsia andeanae, a SFGR of undetermined pathogenicity, has been detected in the United States and Central and South America (2,10). Our findings highlight the need for specific diagnostic tests for SFGR in Mexico that can identify other potential SFGR of public health concern in this country.
Dr. Delgado-de la Mora is a researcher and resident of Pathology at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. His research interests include tickborne diseases, particularly those caused by Rickettsia spp.
References
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–11. DOIPubMedGoogle Scholar
- Allerdice MEJ, Beati L, Yaglom H, Lash RR, Delgado-de la Mora J, Licona-Enriquez JD, et al. Rickettsia parkeri (Rickettsiales: Rickettsiaceae) detected in ticks of the Amblyomma maculatum (Acari: Ixodidae) group collected from multiple locations in southern Arizona. J Med Entomol. 2017;54:1743–9. DOIPubMedGoogle Scholar
- Álvarez-Hernández G, Roldán JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis. 2017;17:e189–96. DOIPubMedGoogle Scholar
- Sánchez-Montes S, López-Pérez AM, Guzmán-Cornejo C, Colunga-Salas P, Becker I, Delgado-de la Mora J, et al. Rickettsia parkeri in Dermacentor parumapertus Ticks, Mexico. Emerg Infect Dis. 2018;24:1108–11. DOIPubMedGoogle Scholar
- Lado P, Nava S. Mendoza-Uribe L, Caceres AG, Delgado-de la Mora J, Lincona-Enriquez JD, et al. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: phenotypic plasticity or incipient speciation? Parasites Vectors. 2018;11:610.
- Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol. 2013;51:314–7. DOIPubMedGoogle Scholar
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89. DOIPubMedGoogle Scholar
- Massung RF, Davis LE, Slater K, McKechnie DB, Puerzer M. Epidemic typhus meningitis in the southwestern United States. Clin Infect Dis. 2001;32:979–82. DOIPubMedGoogle Scholar
- Tzianabos T, Anderson BE, McDade JE. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J Clin Microbiol. 1989;27:2866–8.PubMedGoogle Scholar
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, et al. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol. 2010;76:2689–96. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: March 05, 2019
1These authors contributed equally to this article.
Table of Contents – Volume 25, Number 4—April 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correpondence: Jesús Delgado-de la Mora, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Departamento de Anatomía Patológica, Avenida Vasco de Quiroga 15, Belisario Domínguez, C.P. 14080, Mexico City, Mexico
Top