Volume 25, Number 7—July 2019
Research Letter
Human Enterovirus C105, China, 2017
Cite This Article
Citation for Media
Abstract
We report a case of enterovirus C105 infection in an 11-year-old girl with lower respiratory tract symptoms that was identified through the Respiratory Virus Surveillance System, which covers 30 sentinel hospitals in all 16 districts of Beijing, China. The presence of this virus strain in China confirmed its geographically wide distribution.
Enteroviruses are small, nonenveloped RNA viruses that cause illnesses in humans ranging from mild to severe (1). Fifteen species of enterovirus are known, 7 of which are known to infect humans. These species include enterovirus A–D and rhinovirus A–C (1,2). The newly emerging genotype C105 (EV-C105) represents a novel monophyletic clade of enterovirus C; this strain was identified in 2010 in the Democratic Republic of the Congo (strain 34S) (3,4). EV-C105 cases from Italy (Pavia/8376, Pavia/9095), Romania (ROM31), the United States (USA/OK/2014-19362), New Zealand (strains not available), and Burundi (BU77, BU5) have been identified and characterized, suggesting that the spread of EV-C105 could be wider than previously hypothesized (5). Here, we report a detected case of EV-C105 in an 11-year-old girl with lower respiratory tract symptoms in Beijing, China.
The Beijing Center for Disease Prevention and Control established the Respiratory Virus Surveillance System (RVSS) in 2014. The RVSS tracks patients with respiratory tract infections and pneumonia in 30 sentinel hospitals throughout Beijing. The RVSS is an active system, designed to alert for future outbreaks of respiratory infections (RTIs). To study enterovirus infections, we tested 24,093 clinical specimens (nasopharyngeal swab, sputum, and alveolar lavage fluid) from patients with RTIs that were reported through RVSS during June 2014–December 2017. RVSS classifies persons <14 years of age as children and those >14 years of age as adults. The ages of the reported patients ranged from 8 months to 93 years (median 33.5 years, mean 37.9 years).
We screened all samples using real-time PCR for influenza virus, parainfluenza virus types 1–4, respiratory syncytial virus, coronaviruses (229E, NL63, HKU1, and OC43), metapneumovirus, adenovirus, bocavirus, and enteroviruses (6). Overall, 445 (445/7,122; 6.2%) children and 276 (276/16,971; 1.6%) adults were positive for enterovirus or other respiratory viruses.
We further genotyped enterovirus-positive samples with primers sequentially targeting the viral protein (VP) 1 region (7,8). We obtained a 699-nt amplicon of EV-C105 from a nasopharyngeal swab sample collected at the time of a hospital visit (GenBank accession no. KX910099). The patient was an 11-year-old girl with no underlying disease who was brought to the outpatient clinic of the Beijing Children’s Hospital on May 23, 2016, with an 8-day history of fever (highest temperature 38.7°C), coughing, and difficulty breathing. Blood tests in the clinic showed total leukocyte count 1.41 × 1010 cells/L; neutrophils, 79.2%; lymphocytes, 12.1%; total platelet count, 5.2 × 1011/L; and hemoglobin, 130 g/L. Chest radiographs showed thickness or turbulence in the texture in both lungs, which was diagnosed as pneumonia. She received supportive treatment and received antimicrobial drugs empirically before being sent home the same day. She was not hospitalized during her illness. According to a follow-up survey, she recovered 14 days later. We detected no other respiratory pathogens in this patient.
BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi) of the amplicon (Beijing-R2759) showed that the sequences had 98% identity with the USA/OK/2014–19362 strain (GenBank accession no. KX276189.1) and 91.4% with the reference prototype genotype (EV-C105 strain 34S; accession no. JX514943). Phylogenetic analysis of the VP1 gene performed with MEGA version 6.06 software (http://www.megasoftware.net) showed that Beijing-R2759 belonged to genotype EV-C105.
To further characterize this virus strain, we amplified the genome sequence directly from the nasopharyngeal swab sample using reverse transcription PCR with overlapping primers; we sequenced each amplicon 4 times using the Sanger method. We assembled sequences using Lasergene version 5.01 (DNAStar Inc., https://www.dnastar.com). The genome of Beijing-R2759 (GenBank accession no. MH229997) was 7,316 nt, including 6,618 nt in open reading frame. The EV-C105 polyprotein sequence for this strain shares 96.6%–99.4% amino acid identity with 8 of the EV-C105 sequences in GenBank: 96.6% with accession no. KM880097 (Burundi); 96.8% with accession no. KM880096 (Burundi); 97.7% with accession no. JX393302 (Peru); 98.5% with accession no. JX514943 (Republic of the Congo); 98.6% with accession no. KM880098 (Italy); 98.8% with accession no. KM880099 (Romania); 99.3% with accession no. KX276189 (United States); and 99.4% with accession no. KM880100 (Italy).
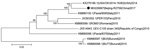
The full length of the Beijing-R2759 VP1 gene was 888 nt. The deduced amino acid sequence in VP1 had 94.9%–100% identity with those from Italy, Peru, Republic of the Congo, and the United States. Alignment results analysis of VP1 aa sequences showed differences between the strains isolated in this study (Met25, Asp138, Ser207) and EV-C105 strain 34S (Val25, Glu138, and Ala207). In this study, we grouped Beijing-R2759 with the strain obtained from the United States in 2014 (Figure). We observed a similar relationship in the phylogenic tree of the VP1 gene. These findings indicate that Beijing-R2759 is closely related to the EV-C105 strain reported in the United States.
Our report confirms that the distribution of EV-C105 is geographically wider than previously believed. A greater awareness of EV-C105 may enable improved detection of this virus (9). In addition, our findings show the utility of the RVSS in assessing the patterns of circulation of enterovirus genotypes and detecting enterovirus outbreaks for the purpose of early warning.
Dr. Li is an assistant researcher in the Institute for Immunization and Prevention of the Beijing Center for Disease Prevention and Control. His research interests include early warning systems and respiratory pathogens.
Acknowledgment
This work was supported by the National Major Science and Technology Project for Control and Prevention of Major Infectious Diseases of China (2017ZX101304), the Beijing Municipal Science and Technology Commission (Z151100003915140), and the Capital Medical Development and Scientific Research Fund (2016-2-3011).
References
- Lukashev AN, Drexler JF, Kotova VO, Amjaga EN, Reznik VI, Gmyl AP, et al. Novel serotypes 105 and 116 are members of distinct subgroups of human enterovirus C. J Gen Virol. 2012;93:2357–62. DOIPubMedGoogle Scholar
- Van Leer-Buter CC, Poelman R, Borger R, Niesters HG. Newly identified enterovirus C genotypes, identified in the Netherlands through routine sequencing of all enteroviruses detected in clinical materials from 2008 to 2015. J Clin Microbiol. 2016;54:2306–14. DOIPubMedGoogle Scholar
- Grard G, Drexler JF, Lekana-Douki S, Caron M, Lukashev A, Nkoghe D, et al. Type 1 wild poliovirus and putative enterovirus 109 in an outbreak of acute flaccid paralysis in Congo, October-November 2010. Euro Surveill. 2010;15:19723. DOIPubMedGoogle Scholar
- Richter J, Tryfonos C, Panagiotou C, Nikolaou E, Koliou M, Christodoulou C. Newly emerging C group enteroviruses may elude diagnosis due to a divergent 5′-UTR. Int J Infect Dis. 2013;17:e1245–8. DOIPubMedGoogle Scholar
- Todd A, Taylor S, Huang QS. Identification of Enterovirus C105 for the first time in New Zealand. Western Pac Surveill Response J. 2015;6:60–2. DOIPubMedGoogle Scholar
- Zhang T, Ren L, Luo M, Li A, Gong C, Chen M, et al. Enterovirus D68-associated severe pneumonia, China, 2014. Emerg Infect Dis. 2015;21:916–8. DOIPubMedGoogle Scholar
- Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–704. DOIPubMedGoogle Scholar
- Oberste MS, Peñaranda S, Rogers SL, Henderson E, Nix WA. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J Clin Virol. 2010;49:73–4. DOIPubMedGoogle Scholar
- Barnadas C, Midgley SE, Skov MN, Jensen L, Poulsen MW, Fischer TK. An enhanced Enterovirus surveillance system allows identification and characterization of rare and emerging respiratory enteroviruses in Denmark, 2015-16. J Clin Virol. 2017;93:40–4. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: May 29, 2019
1These authors contributed equally to this article.
Table of Contents – Volume 25, Number 7—July 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Fang Huang, Beijing Center for Disease Prevention and Control, Institute for Communicable Disease Control and Prevention, No. 16, Hepingli Middle Av, Dongcheng District, Beijing 100013, China
Top