Volume 25, Number 8—August 2019
Research Letter
Zoonotic Virus Seroprevalence among Bank Voles, Poland, 2002–2010
Cite This Article
Citation for Media
Abstract
Bank voles in Poland are reservoirs of zoonotic viruses. To determine seroprevalence of hantavirus, arenavirus, and cowpox virus and factors affecting seroprevalence, we screened for antibodies against these viruses over 9 years. Cowpox virus was most prevalent and affected by extrinsic and intrinsic factors. Long-term and multisite surveillance is crucial.
The most prevalent rodentborne zoonotic viruses in Europe are hantaviruses, lymphocytic choriomeningitis virus (LCMV), cowpox virus (CPXV), and Puumala virus (PUUV) (1). In 2016, a total of 18 countries in Europe reported 2,190 cases of hantavirus disease, mainly caused by PUUV. The occurrence of rodentborne viruses in Poland is not well documented. The first outbreak of hantavirus infections among humans (9 cases) was reported in 2007. During 2012–2016, a total of 79 cases of hantavirus infections were reported in Poland, 55 of them in Podkarpackie Province in 2014 (2). In 2015, a case of human cowpox infection was reported in Poland (3).
We conducted a multisite, long-term study of hantavirus and arenavirus seroprevalence in northeastern Poland. Our objectives were to monitor seroprevalence of LCMV, CPVX, and PUUV in 3 populations of bank voles (Myodes glareolus) from ecologically similar but disparate sites in northeastern Poland and to analyze intrinsic (host sex, host age) and extrinsic (study year, study sites) factors that might affect seroprevalence among these rodent populations.
Study sites were located in the Mazury Lake District region in northeastern Poland (Appendix Figure 1). The sites and methods used for trapping rodents and sampling and processing trapped animals have been described (4). We analyzed serum samples by using an immunofluorescence assay (IFA) (Appendix Figure 2). We diluted serum samples 1:10 in phosphate-buffered saline and tested their reactivity to hantaviruses by using a PUUV IFA, to cowpox viruses by using a CPXV IFA, and to arenaviruses by using an LCMV IFA (5). IFAs were conducted as previously described (6,7). The statistical approach has been comprehensively documented (4).
We tested 652 bank voles and detected antibodies against all 3 viruses. Overall seroprevalence of combined viral infections was 25.9% (95% CI 23.0%–29.1%), but most infections were attributable to CPXV (seroprevalence 25% [95% CI 22.1%–28.2%]). Only 2 voles were LCMV seropositive (0.3% [95% CI 0.2%–0.9%]), and only 5 were PUUV seropositive (0.76% [95% CI 0.4%–1.6%]). We therefore confined further analyses to CPXV.
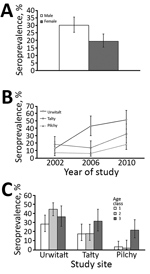
The effect of study year on CPXV seroprevalence (by χ2/d.f.) was highly significant (χ22 31.2; p<0.001); seroprevalence was 2.7 times higher among bank voles sampled in 2010 (36.1% [95% CI 31.7%–40.7%]) than in 2002 (13.1% [7.4%–21.0%]). CPXV seroprevalence also varied markedly among voles from the 3 study sites (χ22 46.84; p<0.001); seroprevalence was highest among voles from Urwitałt (38.4% [95% CI 33.9%–43.1%]) and lower among voles from Tałty (23.0% [19.3%–27.2%]) and Pilchy (10.3% [95% CI 5.7%–17.4%]). CPXV seroprevalence was also significantly affected by the sex of the host (χ21 10.1; p = 0.001) and was 1.5 times higher for male than female voles (Figure, panel A). Seroprevalence increased with host age (χ22 12.73; p = 0.002) and was lowest among voles from age class 1 (immature) (16.0% [95% CI 10.0%–24.1%]) and higher among those from age class 2 (mostly young adults) (27.2% [95% CI 23.2%–31.5%]) and age class 3 (breeding older adults) (30.1% [95% CI 25.9%–34.6%]).
The differences in seroprevalence between sites were also confounded by interaction with study year (year × site × presence/absence of antibodies against CPXV; χ24 12.76; p = 0.012). Seroprevalence increased significantly at all 3 study sites from 2006 to 2010 and was highest in Urwitałt (0.83-fold). The largest seroprevalence increases from 2006 to 2010 were in Tałty (2.35-fold) and Pilchy (2.9-fold) (Figure, panel B).
The pattern of age-related changes in seroprevalence also differed between study sites (site × age × presence/absence of antibodies against CPXV; χ24 = 17.45; p = 0.002) (Figure, panel C). In Urwitałt, the overall seroprevalence was highest among voles in age class 2 (44.5% [95% CI 37.5%–51.8%]), 1.57-fold lower among voles in age class 1, and 1.22-fold lower among voles in age class 3. In Tałty and Pilchy, seroprevalence was highest among voles from age class 3. In Tałty, seroprevalence was 1.8-fold higher among voles in age class 3 compared with voles in other age classes. In Pilchy, seroprevalence among voles in age class 3 was 10.8-fold higher than among voles in age class 2.
Our data show that CPXV was the dominant viral pathogen among bank voles in Poland during the study period, although PUUV and LCMV were also found. Our finding that the highest seroprevalence was among bank voles from Urwitałt complements our previous reports on other pathogens, reflects the importance of extrinsic effects on prevalence, and establishes that the sites from which host populations are sampled is the most influential factor affecting prevalence (4).
Our results provide additional information about the role of bank voles in Poland as infectious virus reservoirs. Although short-term cross-sectional studies are useful as a starting point (8), to obtain a comprehensive ecologic picture, long-term monitoring (several years and preferably a decade or longer) and a multisite approach are crucial. Identifying rodent species that can serve as reservoirs for zoonotic disease viruses and predicting regions where new outbreaks are most likely to happen are crucial steps for preventing and minimizing the extent of zoonotic disease among humans (9).
Dr. Grzybek is a parasitologist holding the position of assistant professor in the Department of Tropical Parasitology, Medical University of Gdansk, Poland. His research interests include epidemiology and ecology of macroparasites and microparasites in rodents, especially bank voles. He is also interested in host–parasite interactions and intrinsic and extrinsic factors that influence these relationships.
Acknowledgments
We thank the University of Nottingham, Warsaw University, and the Medical University of Gdansk for financial support. M.G. thanks Alicja Rost and Ewa Zieliniewicz for their assistance in the laboratory.
This research received support from Sigrid Jusélius Foundation, Helsinki, and the Natural Resources Institute Finland. J.M.B. received support from the Royal Society, the British Ecological Society, and the Grabowski Fund. A.B. received support from the Polish State Committee for Scientific Research and the British Council’s Young Scientist Programme. M.G. received support from the Ministry of Science and Higher Education in Poland, Fellowship for Outstanding Scientists (428/STYP/11/2016).
References
- Kallio-Kokko H, Uzcategui N, Vapalahti O, Vaheri A. Viral zoonoses in Europe. FEMS Microbiol Rev. 2005;29:1051–77. DOIPubMedGoogle Scholar
- European Centre for Disease Prevention and Control. Hantavirus infection. Annual epidemiological report for 2016 [cited 2019 June 20]. https://ecdc.europa.eu/sites/portal/files/documents/hantavirus-infection-annual-epidemiological-report-2016.pdf
- Świtaj K, Kajfasz P, Kurth A, Nitsche A. Cowpox after a cat scratch - case report from Poland. Ann Agric Environ Med. 2015;22:456–8. DOIPubMedGoogle Scholar
- Grzybek M, Bajer A, Bednarska M, Al-Sarraf M, Behnke-Borowczyk J, Harris PD, et al. Long-term spatiotemporal stability and dynamic changes in helminth infracommunities of bank voles (Myodes glareolus) in NE Poland. Parasitology. 2015;142:1722–43. DOIPubMedGoogle Scholar
- Hedman K, Vaheri A, Brummer-Korvenkontio M. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet. 1991;338:1353–6. DOIPubMedGoogle Scholar
- Kallio-Kokko H, Laakkonen J, Rizzoli A, Tagliapietra V, Cattadori I, Perkins SE, et al. Hantavirus and arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiol Infect. 2006;134:830–6. DOIPubMedGoogle Scholar
- Pelkonen PM, Tarvainen K, Hynninen A, Kallio ERK, Henttonen K, Palva A, et al. Cowpox with severe generalized eruption, Finland. Emerg Infect Dis. 2003;9:1458–61. DOIPubMedGoogle Scholar
- Sadkowska-Todys M, Dudek-Godeau D, Kamińska S, Baumann-Popczyk A, Czerwiński M, Kucharczyk B, et al. Occurrence and maintenance of hantavirus infections among rodent populations in their natural habitat—results of a field study from Podkarpackie province, Poland 2010-2012. Przegl Epidemiol. 2015;69:283–8, 395–9.PubMedGoogle Scholar
- Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. Proc Natl Acad Sci U S A. 2015;112:7039–44. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: July 06, 2019
1These authors contributed equally to this article.
Table of Contents – Volume 25, Number 8—August 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Maciej Grzybek, Medical University of Gdansk, Department of Tropical Parasitology, Powstania Styczniowego 9B, 81-519 Gdynia, Poland
Top