Volume 26, Number 11—November 2020
Research Letter
Saliva Alternative to Upper Respiratory Swabs for SARS-CoV-2 Diagnosis
Figure
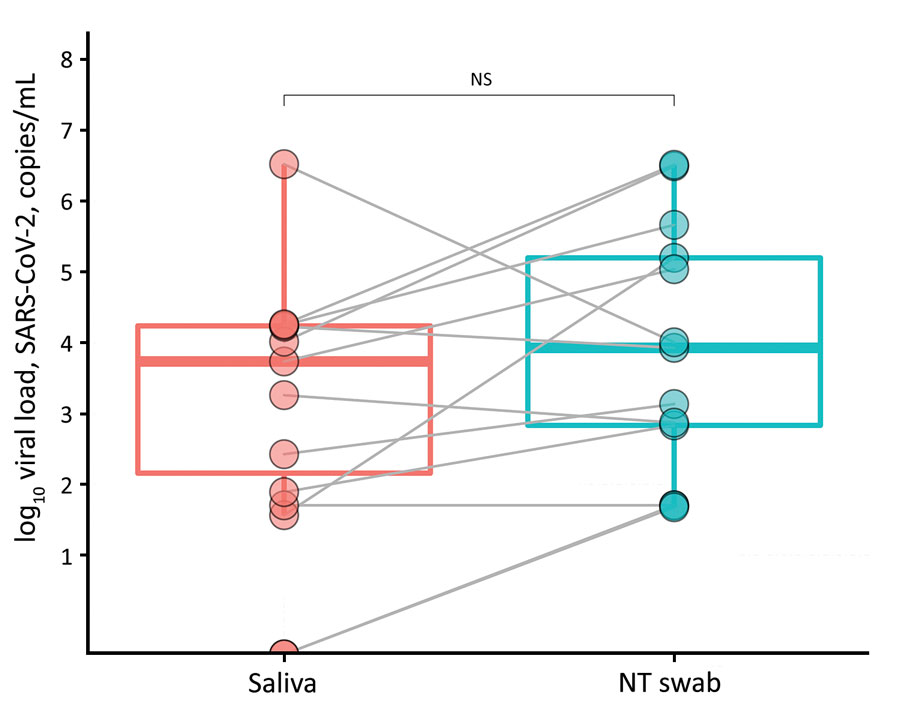
Figure. Viral load (copies/mL) of SARS-CoV-2 RNA recovered from paired saliva samples and nasal and throat swab specimens from 14 patients with coronavirus disease, United Kingdom, 2020. Viral loads are shown on a logarithmic scale. NS, not significant; NT, nasal and throat; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Page created: August 10, 2020
Page updated: October 19, 2020
Page reviewed: October 19, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.