Volume 26, Number 12—December 2020
Research
Risk for Hepatitis E Virus Transmission by Solvent/Detergent–Treated Plasma
Figure
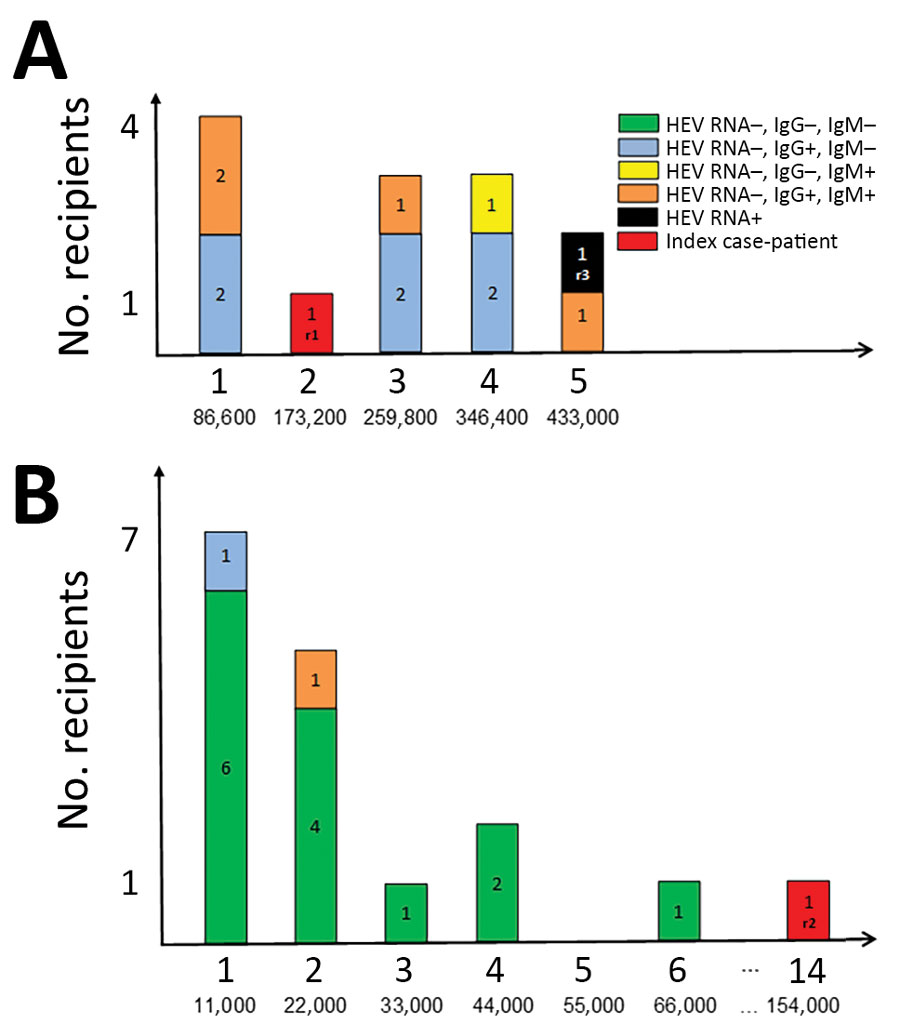
Figure. Transfused, HEV-infected solvent/detergent–treated plasma and recipient HEV status. A) Lot A; B) lot B. Top values along each x-axis indicate the number of solvent/detergent–treated plasma units transfused per recipient; bottom values indicate HEV viral load (IU/recipient). HEV, hepatitis E virus.
Page created: August 28, 2020
Page updated: November 19, 2020
Page reviewed: November 19, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.