Volume 26, Number 8—August 2020
Research Letter
Cryptosporidium baileyi Pulmonary Infection in Immunocompetent Woman with Benign Neoplasm
Abstract
Cryptosporidium baileyi, a bird-specific parasite, infects gastrointestinal, pulmonary, and urinary tracts of its host. We report on a C. baileyi infection associated with pulmonary hamartoma in an immunocompetent patient in Poland. Further work is needed to investigate the association between Cryptosporidium infections and tumors.
Cryptosporidium is a global protozoan parasite infecting wild, agricultural, and domestic vertebrates, and humans. Most infections lead to a gastrointestinal illness characterized by diarrhea (1). In addition, in birds, C. baileyi and C. avium parasites cause pulmonary infection (2). In humans, respiratory cryptosporidiosis is mostly associated with immunodeficiency, but several cases in immunocompetent children with intestinal cryptosporidiosis also have been reported (3,4). We describe a C. baileyi infection associated with a lung hamartoma in an immunocompetent patient.
The Human Research Ethics Committee of Wroclaw Medical University (Wroclaw, Poland) approved the use of diagnostic samples and corresponding patient data for this study (permit no. KB648/2014). The patient provided written informed consent for conduct of the studies and publication of the results.
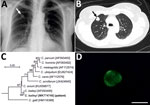
In June 2015, a 51-year-old immunocompetent woman living in a rural area of Poland was admitted to a hospital with a suspected spinal injury. In addition to confirming the spinal injury, a solitary pulmonary nodule (SPN), 1.3–1.8 cm, in the third segment of the right lung upper lobe was detected by chest radiography and computed tomography (Figure, panels A, B). The lesion was of high density and had well-defined borders. The patient was referred to the Department of Pulmonology and Lung Cancers of Wroclaw Medical University for a nodule differential diagnosis. A bronchoscopy performed in July 2015 included biopsy samples for histology and cytology and a bronchial washings (BW) sample for acid-fast bacilli examination. We did not detect any changes in the bronchial trees, vocal cords, or trachea. Histologic and cytologic examination showed low cell count material, and the cells of the nodule lacked features of malignant process. The BW was negative for acid-fast bacilli. A second chest radiograph and bronchoscopy performed before surgery in September 2015 to remove the SPN showed a normal bronchial tree without anatomic changes and a slight increase in size of the SNP (1.5–2.0 cm). The SPN was removed by wedge resection in video-assisted thoracoscopic surgery after stabilization of the spine.
Before and during hospitalization, the patient was in good physical condition, afebrile, and without signs or symptoms of pulmonary and gastrointestinal infection. She reported no chronic diarrhea or cough in recent years. Blood count data, acid-base balance, gasometry, and spirometry did not deviate from mean values. She had no palpable lymphadenopathy. Except for the SPN, medical history was unremarkable. The patient was not undergoing immunosuppressive treatment and did not have an autoimmune disease. She was discharged 1 week after surgery in good general condition.
A paraffin-embedded tissue sample from the removed mass was diagnosed as a bronchial hamartoma. The BW was tested for pulmonary pathogens using individual conventional PCR (Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella pneumophila, Cryptosporidium, Encephalitozoon, and Enterocytozoon) and real-time PCR (Pneumocystis jirovecii). To identify Cryptosporidium, we extracted DNA from the BW and amplified a partial sequence of the small-subunit rRNA of Cryptosporidium (5) and sequenced it in triplicate. We inferred relationships to sequences from known species/genotypes from maximum-likelihood phylogenies (MEGA X, https://www.megasoftware.net). Bootstrap support for branching was based on 1,000 replications. A BW smear was labeled with genus-specific fluorescein isothiocyanate–conjugated antibodies (indirect fluorescent antibody Crypto cel; Cellabs Pty Ltd., https://www.cellabs.com.au).
The small-subunit sequence (GenBank accession no. MK774740) for the patient isolate was identical to that of a C. baileyi isolate from chickens (GenBank accession no. AF093495) (Figure, panel C). Microscopic examination of the BW smear showed oocysts measuring 5.2–5.5 μm with typical green fluorescence after labeling with FITC-conjugated anti-Cryptosporidium oocyst wall antibody (Figure, panel D).
We report an unusual case of human respiratory C. baileyi infection in an immunocompetent woman with hamartoma. Previously, a presumptive C. baileyi infection had been reported in the lungs, trachea, larynx, esophagus, whole intestine, gallbladder, and urinary tract of an immunodeficient man with HIV (6). Although not confirmed by sequence analysis, the finding that oocysts were infective for chickens supports the identification.
Immunocompetence is a critical determinant of the clinical course of Cryptosporidium infection. Intestinal cryptosporidiosis can be devastating to immunodeficient persons (7) and can involve extraintestinal sites (6). Pulmonary cryptosporidiosis in immunocompetent humans typically manifests as a cough and dyspnea, and concurrent intestinal infection is frequently reported (3,4). Consistent with this, the overwhelming majority of respiratory cases in humans have been caused by C. parvum and C. hominis, the major causes of intestinal cryptosporidiosis (3,4,8). Respiratory Cryptosporidium infection in birds is mostly asymptomatic but can result in high rates of death in association with other etiologic agents (2). The patient in this study did not report direct contact with birds but might have been exposed to wild bird excretions occupationally through work in an orchard. The hamartoma could have created an environment that supported the C. baileyi infection. C. meleagridis parasites were recently detected around a colon adenocarcinoma in an immunocompetent patient who, similarly, did not have symptoms typically associated with cryptosporidiosis (9). Alternatively, experimental evidence suggests that Cryptosporidium parasites can cause neoplastic changes in immunocompromised animals and in cells cultured in vitro, consistent with a role in carcinogenesis (10).
Dr. Kopacz is a research scientist at the Department of Biology and Medical Parasitology, Wroclaw Medical University. Her research focuses on opportunistic parasites, including microsporidia, Cryptosporidium spp., and Pneumocystis jirovecii in humans and animals.
Acknowledgment
This work was supported by the Grant for Young Scientist of Wroclaw Medical University, Poland (STM.A060.17.038) and the Czech Science Foundation (18-12364S).
References
- Plutzer J, Lassen B, Jokelainen P, Djurković-Djaković O, Kucsera I, Dorbek-Kolin E, et al. Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Euro Surveill. 2018;23:1–23. DOIPubMedGoogle Scholar
- Nakamura AA, Meireles MV. Cryptosporidium infections in birds—a review. Rev Bras Parasitol Vet. 2015;24:253–67. DOIPubMedGoogle Scholar
- Sponseller JK, Griffiths JK, Tzipori S. The evolution of respiratory Cryptosporidiosis: evidence for transmission by inhalation. Clin Microbiol Rev. 2014;27:575–86. DOIPubMedGoogle Scholar
- Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, et al. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clin Infect Dis. 2010;50:1366–72. DOIPubMedGoogle Scholar
- Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, Shulaw W, et al. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol. 1999;65:3386–91. DOIPubMedGoogle Scholar
- Ditrich O, Palkovic L, Stĕrba J, Prokopic J, Loudová J, Giboda M. The first finding of Cryptosporidium baileyi in man. Parasitol Res. 1991;77:44–7. DOIPubMedGoogle Scholar
- Mead JR, Arrowood MJ. Treatment of cryptosporidiosis. In: Cacciò SM, Widmer G, editors. Cryptosporidium: parasite and disease. Heidelberg (Germany): Springer: 2014. p. 455–88.
- Reina FTR, Ribeiro CA, Araújo RS, Matté MH, Castanho REP, Tanaka II, et al. INTESTINAL AND PULMONARY INFECTION BY Cryptosporidium parvum IN TWO PATIENTS WITH HIV/AIDS. Rev Inst Med Trop São Paulo. 2016;58:21. DOIPubMedGoogle Scholar
- Kopacz Ż, Kváč M, Karpiński P, Hendrich AB, Sąsiadek MM, Leszczyński P, et al. The first evidence of Cryptosporidium meleagridis infection in a colon adenocarcinoma from an immunocompetent patient. Front Cell Infect Microbiol. 2019;9:35. DOIPubMedGoogle Scholar
- Benamrouz S, Conseil V, Chabé M, Praet M, Audebert C, Blervaque R, et al. Cryptosporidium parvum-induced ileo-caecal adenocarcinoma and Wnt signaling in a mouse model. Dis Model Mech. 2014;7:693–700. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: July 14, 2020
Table of Contents – Volume 26, Number 8—August 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Żaneta Kopacz, Department of Biology and Medical Parasitology, Wrocław Medical University, ul. J. Mikulicza-Radeckiego 9, 50-367 Wrocław, Poland
Top