Volume 26, Number 8—August 2020
Synopsis
Investigation and Serologic Follow-Up of Contacts of an Early Confirmed Case-Patient with COVID-19, Washington, USA
Figure 1
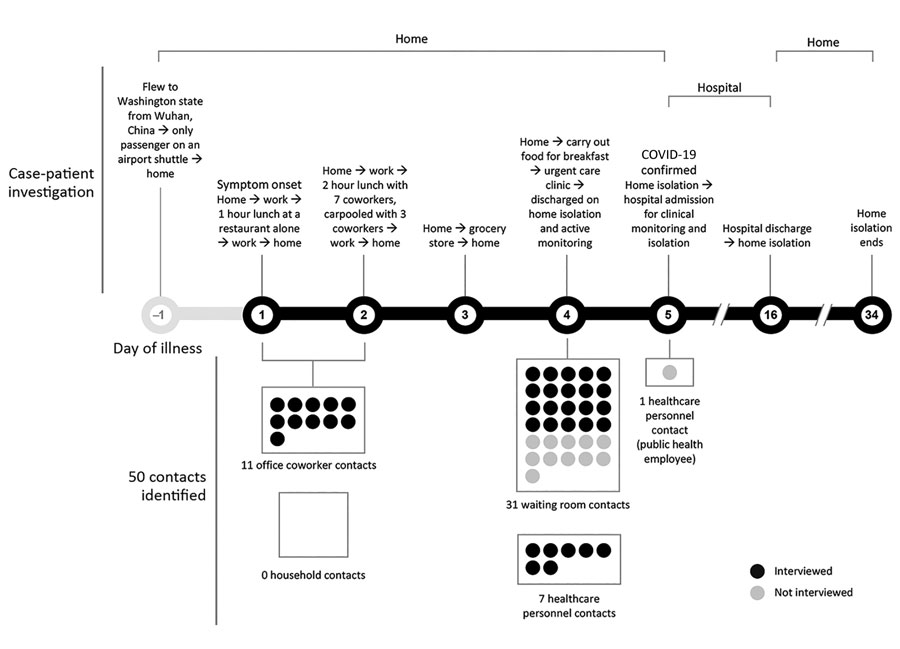
Figure 1. Case-patient investigation and contact identification during the investigation of an early confirmed US COVID-19 case, Washington, USA, 2020. The case-patient was asymptomatic when he arrived home from Wuhan, China. The next day, he developed a cough (day 1), followed by chills (day 2) and a subjective fever (day 3). When he arrived at the urgent care clinic (day 4), he was given a facemask and sat in the waiting room for ≈20 minutes. He was evaluated in a standard examination room, and received a chest radiograph in a radiology room down the hallway from the exam room. The case-patient was identified as meeting the Centers for Disease Control and Prevention (CDC) criteria at the time for a person under investigation for COVID-19, and specimens (nasopharyngeal and oropharyngeal swabs and serum samples) were collected for testing (6). He was clinically stable and discharged home pending SARS-CoV-2 test results. When COVID-19 was confirmed (day 5), the case-patient was admitted to a hospital for observation and isolation. After 11 days, he was discharged to home isolation until 2 negative sets of nasopharyngeal and oropharyngeal specimens were obtained >24 hours apart, in accordance with CDC guidance at the time (7). Persons exposed during transient interactions, such as restaurant waitstaff and persons encountered at the grocery store, were not considered community contacts. COVID-19, coronavirus disease.
References
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. DOIPubMedGoogle Scholar
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al.; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel; 2020 [cited 2020 Mar 30]. https://www.fda.gov/media/134922/download
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3. DOIPubMedGoogle Scholar
- Trivedi S, Miao C, Al-Abdallat MM, Haddadin A, Alqasrawi S, Iblan I, et al. Inclusion of MERS-spike protein ELISA in algorithm to determine serologic evidence of MERS-CoV infection. J Med Virol. 2018;90:367–71. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update and interim guidance on outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China; 2020 Jan 17 [cited 2020 Apr 5]. https://emergency.cdc.gov/han/han00426.asp
- Kujawski SA, Wong KK, Collins JP, Epstein L, Killerby ME, Midgley CM, et al.; COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020.PubMedGoogle Scholar
- Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, et al.; Investigation Team. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25:
2000094 . DOIPubMedGoogle Scholar - Burke RM, Midgley CM, Dratch A, Fenstersheib M, Haupt T, Holshue M, et al. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January–February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245–6. DOIPubMedGoogle Scholar
- Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, et al.; Illinois COVID-19 Investigation Team. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–44. DOIPubMedGoogle Scholar
- Scott SE, Zabel K, Collins J, Hobbs KC, Kretschmer MJ, Lach M, et al. First mildly ill, non-hospitalized case of coronavirus disease 2019 (COVID-19) without viral transmission in the United States—Maricopa County, Arizona, 2020. Clin Infect Dis. 2020 Apr 2 [Epub ahead of print].
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) cases in the U.S.; 2020 [cited 2020 Apr 20]. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–9. DOIPubMedGoogle Scholar
- Cheng VC, To KK, Tse H, Hung IF, Yuen KY. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin Microbiol Rev. 2012;25:223–63. DOIPubMedGoogle Scholar
- Ng K, Poon BH, Kiat Puar TH, Shan Quah JL, Loh WJ, Wong YJ, et al. COVID-19 and the risk to health care workers: a case report. [ Mar 16 ] [Epub ahead of print]. Ann Intern Med. 2020;
L20-0175 . DOIPubMedGoogle Scholar - Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;
ciaa344 . DOIPubMedGoogle Scholar - To KK-W, Tsang OT-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–74. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.