Volume 26, Number 8—August 2020
Research Letter
Delayed Laboratory Response to COVID-19 Caused by Molecular Diagnostic Contamination
Figure
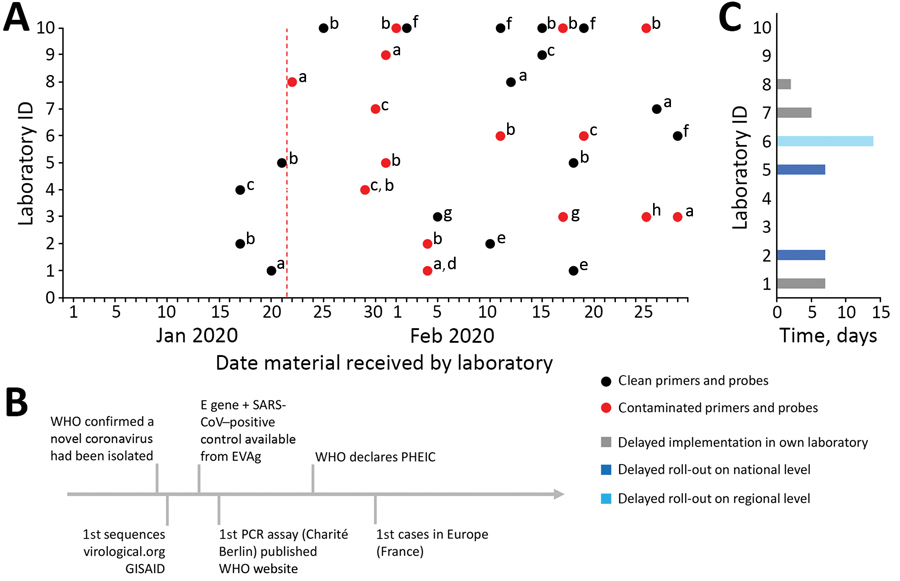
Figure. Timeline and extent of product and molecular diagnostic contamination issues in 10 laboratories in Europe during delayed laboratory response to COVID-19. A) Contamination status of commercially ordered primers and probes for molecular detection of SARS-CoV-2 based on Corman et al. (2). Red vertical dotted line indicates starting date of laboratories in Europe receiving contaminated commercial primers and probes. The letters a–h are unique identifiers for the 8 companies that produced the materials. B) Timeline of simultaneous hallmark events in the SARS-CoV-2 outbreak. C) Delay of implementation of SARS-CoV-2 diagnostic test in laboratories and delay of national or regional roll-out schemes per laboratory. Laboratories that indicated no delay had access to noncontaminated material from previous orders or cooperated with another laboratory. COVID-19, coronavirus disease; E, envelope; EVAg, European Virus Archive Global; GISAID, Global Initiative on Sharing All Influenza Data (http://gisaid.org); ID, identification; PHEIC, public health emergency of international concern; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
References
- Reusken CB, Broberg EK, Haagmans B, Meijer A, Corman VM, Papa A, et al. On Behalf Of Evd-LabNet And Erli-Net. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Euro Surveill. 2020;•••:25.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:25. DOIPubMedGoogle Scholar
- World Health Organization. Novel coronavirus. (2019-nCoV) technical guidance: laboratory guidance, 2020 [cited 2020 Mar 3]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance
- European Centre for Disease Prevention and Control. Questions and answers regarding laboratory topics on SARS-CoV-2, 2020 [cited 2020 May 4]. https://www.ecdc.europa.eu/en/all-topics-z/coronavirus/threats-and-outbreaks/covid-19/laboratory-support/questions
- International Gene Synthesis Consortium. 2020 [cited 2020 Mar 29]. https://genesynthesisconsortium.org
1These authors contributed equally to this article.