Volume 27, Number 10—October 2021
Research Letter
Emergomyces orientalis Emergomycosis Diagnosed by Metagenomic Next-Generation Sequencing
Cite This Article
Citation for Media
Abstract
Emergomyces is a newly described dimorphic fungus genus; it may cause fatal infections in immunocompromised patients, but diagnosis is often delayed. We report a case of disseminated emergomycosis caused by the novel species Emergomyces orientalis in a kidney transplant recipient from Tibet. Infection was diagnosed early by metagenomic next-generation sequencing.
Emergomycosis (formerly called emmonsiosis) is an emerging dimorphic fungal disease, usually caused by Emergomyces pasteurianus or Es. africanus, usually disseminated and commonly identified and fatal in immunocompromised patients, especially HIV-positive patients from South Africa (1,2). Diagnosis of emergomycosis is often delayed, and best clinical practices for diagnosing and treating organ transplant recipients are lacking. Five species with different geographic distributions have been described: Es. pasteurianus, Es. africanus, Es. canadensis, Es. europaeus, and Es. orientalis. Globally, the only case of Es. orientalis infection, reported in China in 2017, was initially misdiagnosed as disseminated cryptococcosis (3). We report another case of Es. orientalis infection involving lung and soft tissue damage that was diagnosed early and accurately and treated precisely.
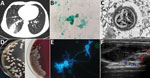
A 41-year-old man from Tibet who had received a kidney transplant 6 years earlier was admitted to a hospital with a 1-month history of progressive right lower chest pain and mild cough with a small amount of sputum. He was taking tacrolimus, mycophenolate mofetil, and prednisone. He was a herder caring for sheep, horses, and dogs. We noted reduced breath sounds in his lower right lung; chest computed tomography images indicated pneumonia (Figure, panel A). A bronchoalveolar lavage fluid smear revealed yeast-like fungi on both Gram staining and Grocott-Gomori methenamine silver staining (Figure, panel B). Because pulmonary cryptococcosis was suspected, fluconazole (400 mg 1×/d) was initiated. Results of a cryptococcal antigen lateral flow immunoassay (IMMY, https://www.immy.com) was negative, but a Platelia Aspergillus antigen immunoenzymatic sandwich microplate assay (Bio-Rad, https://www.bio-rad.com) resulted in an unexpectedly high level (6.42 [reference 0.00–0.49] signal:cutoff ratio). After 1 week of ineffective empirically prescribed treatment, we had a lung biopsy performed. Electron microscopy revealed yeast cells in a unique form, measuring ≈3 μm, scattered in necrotizing granulomas (Figure, panel C). Metagenomic next-generation sequencing (mNGS) of fresh tissue indicated Es. orientalis (sequence reads 143;, Illumina NextSeq 550 platform, https://www.illumina.com; Appendix Figure 1). We initiated oral itraconazole (200 mg 2×/d) immediately and decreased tacrolimus dosage according to its plasma concentration. Finally, we isolated the pure Es. orientalis strain (Figure, panel D). Specific secondary, α-shaped conidiophores clearly indicated Emergomyces (Figure, panel E). Es. orientalis was confirmed by PCR amplification targeting the rDNA internal transcribed spacer region followed by BLAST sequence comparison (https://blast.ncbi.nlm.nih.gov/Blast.cgi; GenBank accession no. NR_148064.1; coverage 96%, identity 99.33%) (Appendix Figure 2).
During treatment, the patient had intermittent mild fever and an acne-like rash on his chin, and a small new pulmonary lesion developed in the right upper lobe. Repeated blood cultures were all negative. We prescribed oral posaconazole (400 mg 2×/d) after determining a MIC of 0.008 μg/mL (Appendix Table). Later, the lung lesions partially resolved, but we found a painful soft tissue abscess (55 × 15 × 30 mm) on the right side of his waist (Figure, panel F) from which we drained purulent grayish-green fluid. We again cultured Es. orientalis. Therefore, we added flucytosine (1,000 mg 3×/d) and withdrew tacrolimus and mycophenolate mofetil for 1 month. After 6 months of recurrent hospitalization, we discharged the patient with a diagnosis of disseminated emergomycosis. Six months after discharge, he remained stable. We found no similarly infected or epidemiologically linked person or animal.
Previously, a retrospective study from southern Africa assessed 54 patients with disseminated emergomycosis, of whom 94% were co-infected with HIV; 96% had skin involvement, 88% had lung involvement, 44% received an incorrect diagnosis, and 48% died (4). In this case, we initially identified Es. orientalis infection using mNGS, a 1-step, culture-independent method for detecting all pathogens from 1 specimen (5). Although research validating mNGS assays in clinical practice is very limited, challenging cases diagnosed by mNGS have been published and expert consensus has begun to recommend mNGS for diagnosing challenging cases in immunocompromised patients (6,7). Therefore, we recommend using mNGS to diagnose challenging emergomycosis cases.
This case showed that treatment with posaconazole combined with flucytosine is effective in organ transplant recipients with disseminated emergomycosis caused by Es. orientalis. Although amphotericin B deoxycholate is more effective than triazoles for improving emergomycosis survival rate (71% vs. 33%) (4), we could not prescribe it for our patient because of nephrotoxicity. Similar to the earlier reported case of Es. orientalis infection, in which type 2 diabetes was the only identified cause of immunodeficiency (3), fluconazole was ineffective in vivo in our patient. Previously, 3 cases in China of Es. pasteurianus (formerly Emmonsia pasteuriana) infection with or without renal transplantation have also been reported (8–10).
Further research is needed to determine whether kidney transplantation is associated with Es. orientalis infection and risk for emergomycosis. In conclusion, clinicians need to become more aware of emergomycosis because of its common misdiagnosis and high death rate.
Dr. He and Dr. Quan are medical students at the Center for Infectious Diseases, West China Hospital of Sichuan University in Chengdu, China. Their research interests are pathogen detection and microbial resistance.
Acknowledgments
We thank Liubo Xiong and Yuling Xiao for their help.
This study was supported by Sichuan Science and Technology program, China (2018HH0031) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (2017-046).
References
- Schwartz IS, Govender NP, Sigler L, Jiang Y, Maphanga TG, Toplis B, et al. Emergomyces: The global rise of new dimorphic fungal pathogens. PLoS Pathog. 2019;15:
e1007977 . DOIPubMedGoogle Scholar - Rooms I, Mugisha P, Gambichler T, Hadaschik E, Esser S, Rath PM, et al. Disseminated emergomycosis in a person with HIV infection, Uganda. Emerg Infect Dis. 2019;25:1750–1. DOIPubMedGoogle Scholar
- Wang P, Kenyon C, de Hoog S, Guo L, Fan H, Liu H, et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses. 2017;60:310–9. DOIPubMedGoogle Scholar
- Schwartz IS, Govender NP, Corcoran C, Dlamini S, Prozesky H, Burton R, et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015;61:1004–12. DOIPubMedGoogle Scholar
- Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66:778–88. DOIPubMedGoogle Scholar
- Editorial Board of the Chinese Journal of Infectious Diseases. Clinical practice expert consensus for the application of metagenomic next generation sequencing [in Chinese]. Chin J Infect Dis. 2020;38:681–9.
- Clinical Microbiology Group of Chinese Society of Laboratory Medicine, Clinical Microbiology Group of Chinese Society of Microbiology and Immunology, Society of Clinical Microbiology and Infection of China International Exchange and Promotion Association for Medical and Healthcare. Chinese expert consensus on metagenomics next-generation sequencing application on pathogen detection of infectious diseases [in Chinese]. Chin J Lab Med. 2021;44:107–20.
- Feng P, Yin S, Zhu G, Li M, Wu B, Xie Y, et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J Dermatol. 2015;42:1179–82. DOIPubMedGoogle Scholar
- Tang XH, Zhou H, Zhang XQ, Han JD, Gao Q. Cutaneous disseminated emmonsiosis due to Emmonsia pasteuriana in a patient with cytomegalovirus enteritis. JAMA Dermatol. 2015;151:1263–4. DOIPubMedGoogle Scholar
- Chik KK, To WK. Autochthonous Emergomyces pasteurianus pneumonia in an immunocompromised patient in Hong Kong: a case report. Hong Kong Med J. 2020;26:446–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: August 31, 2021
1These authors contributed equally to this article.
Table of Contents – Volume 27, Number 10—October 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Xiaohui Wang, Center for Infectious Diseases, West China Hospital of Sichuan University, Wuhouqu Guoxuexiang 37#, Chengdu 610041, China
Top