Volume 27, Number 12—December 2021
Research Letter
Limited and Short-Lasting Virus Neutralizing Titers Induced by Inactivated SARS-CoV-2 Vaccine
Figure
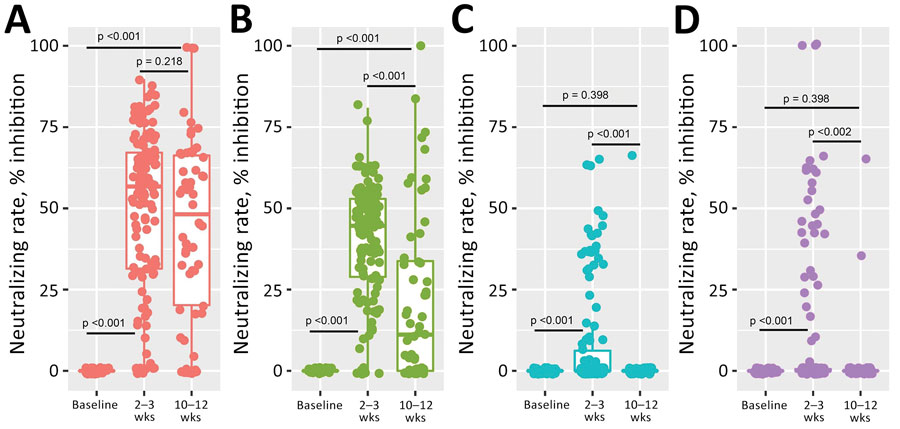
Figure. Results of in vitro testing by microneutralization assay of CoronaVac-induced neutralizing A) Wild-type strain and B) Alpha-, C) Beta-, and D) Delta-variant SARS-CoV-2 antibodies (n = 207). Overall vaccine-induced neutralizing antibodies shown at baseline, 2–3 weeks, and 10–12 weeks after second dose. Differences in mean inhibition rate were compared based on blood collection times. p value <0.05 indicates statistical significance.
Page created: September 16, 2021
Page updated: November 21, 2021
Page reviewed: November 21, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.