Volume 27, Number 4—April 2021
Dispatch
Fatal Case of Crimean-Congo Hemorrhagic Fever Caused by Reassortant Virus, Spain, 2018
Abstract
In August 2018, a fatal autochthonous case of Crimean-Congo hemorrhagic fever was confirmed in western Spain. The complete sequence of the viral genome revealed circulation of a new virus because the genotype differs from that of the virus responsible for another case in 2016. Practitioners should be alert to possible new cases.
A fatal case of Crimean-Congo hemorrhagic fever (CCHF) detected in Spain in 2018 was caused by a different genotype, a reassortant virus, than the genotype of a previous case detected in 2016. This unexpected variability contrasts with the situation in other CCHF-endemic countries. Because CCHF is a zoonotic disease and animal migratory routes between Europe and Africa usually pass through Spain, data about genetic sequences are crucial for monitoring infections in humans, developing suitable detection tools, and providing information about the dynamics of virus circulation and spread.
On July 31, 2018, a 74-year-old man sought care at Nuestra Señora de Sonsoles Hospital (Ávila, Spain) with fever (39.2°C), pain in the sacroiliac area, chills, shivering, and a feeling of dizziness without loss of consciousness. No relevant physical findings or analytical parameters were detected (Table 1). While in the hospital, the patient remained stable and in good general condition. He was discharged for observation at home, afebrile, with a diagnosis of febrile syndrome with bacteremia and a prescription of amoxicillin/clavulanic acid and instructions to take acetaminophen if fever redeveloped.
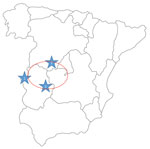
On August 4, the man returned to the hospital with general discomfort and no improvement. He reported increased stools that he (a retired physician) assumed were associated with the antimicrobial drug and reported probably having been bitten by a tick while participating in boar hunting on July 24 in Helechosa de los Montes (Badajoz, Spain) (Figure 1). The patient had skinned the boar and came into close contact with abundant blood. Physical examination on his return to the hospital was unremarkable, but because of persistent symptoms and the appearance of petechiae and thrombocytopenia, the man was hospitalized for laboratory testing (Table 1) and imaging.
On August 7, infection with CCHF virus (CCHFV) was considered. The patient progressively worsened and died at the end of the day. On August 8, a blood sample was collected into an EDTA tube and sent to the National Center for Microbiology (Madrid, Spain) for CCHFV diagnostic testing. The sample was inactivated in the Biosafety Level 3 facility by using a QIAamp viral RNA kit (QIAGEN, https://www.qiagen.com) and after addition of ethanol was sent to the Biosafety Level 2 facility. Diagnostic testing was performed by using a RealStar CCHFV RT-PCR Kit 1.0 (Altona Diagnostics, https://altona-diagnostics.com), and CCHFV infection was confirmed by 2 methods: reverse transcription PCR (1) (slightly modified to incorporate an internal control for amplification) and a nested reverse transcription PCR (2). Results were further confirmed by a World Health Organization Collaborating Center (Public Health England, London, UK).
Molecular and serologic virus detection testing was also performed on additional serum samples. At 6 days after symptom onset, the patient’s viral load (standards kindly provided by Altona Diagnostics) was >108 copies/mL. Specific IgG and IgM against CCHFV were detected by using a commercial indirect immunofluorescence assay (Crimean-Congo Fever Virus Mosaic 2 IFA; Euroimmun, https://www.euroimmun.com), according to the manufacturer’s instructions (Table 2). IgM was detected on day 6 and IgG on day 7, both at very low titers.
Sequencing of small (S), medium (M), and large (L) segments was performed by using primers previously described (3). Complete genome sequences were obtained (GenBank accession nos. MN689738 [S segment], MN689740 [M segment], and MN689741 [L segment]).
Phylogenetic analyses with neighbor-joining and Bayesian (Figure 2) approaches that used MEGA7 (https://www.megasoftware.net) and the Beauti/Beast 1.75 package (https://beast.community) programs show similar results. The strain, Badajoz 2018, belongs to genotype III if the L and M segments are analyzed; however, the S segment is closely related to sequences of genotype IV and shares the highest identity with the strains BT 958 (92.62%) from Central African Republic and IbAn7620 (92.58%) from Nigeria.
Phylogenetic analysis of the virus responsible for a fatal case of CCHF in 2018 showed reassortment, indicating a new CCHFV circulating in Spain. The patient was probably infected by a tick bite obtained while hunting. The incubation period for this patient was longer (7 days) than that typical after a tick bite (1–3 days) (6); however, the patient also participated in skinning the boar. The geographic location of the hunting site is very close to a natural park, bordering regions where the CCHFV genome has been detected in ticks (7) (Figure 1). After returning home, the patient felt ill, but CCHFV was not suspected until 7 days after symptoms appeared, just before he died. No specific treatment was administered. Despite the hospital being located within the region where the first case of CCHFV in Spain was detected, clinician awareness was not high enough to suspect CCHFV infection, partially because the patient diverted attention away from his possible contact with ticks or infected animals. The analytical parameters and the microbiological data are in accordance with described parameters for CCHFV in patients who have died (8), although partial thromboplastin time (and not prothrombin time) was altered in the final hemorrhagic phase, in contrast with parameters for the 2016 CCHF patient in Spain (2).
Sequence analysis revealed circulation of a CCHFV very different genetically than the one previously described in Spain in humans or ticks (2,3,9,10). Complete sequences of viruses detected in humans (2016) and in ticks (2014) have indicated circulation of genotype III viruses, but the virus detected in 2018 is a reassortant in the S segment. Badajoz 2018 L and M segments group within genotype III (with sequences quite different than other sequences from Spain) and the S segment is similar to genotype IV. This S segment is close to the IbAn 7620 strain, isolated from the serum of a goat in 1965 in Nigeria, and the BT 958 strain from the Central African Republic, detected in 1975 and considered by Lukashev et al. (11) as an outlier of genotype IV. Genotype IV is formed by 2 genetic lineages, Asia 1 and Asia 2. Because the differences of Badajoz 2018 and related sequences with the Asia strains of genotype IV are remarkable, obtaining more related sequences from Spain or Africa may enable us to split this genotype into other genetic lineages by defining new genetic groups, probably with an origin in Africa. To date, this new genetic lineage contains sequences from 3 geographic regions detected 3 times.
The sequencing results showing 2 virus genetic lineages circulating in Spain indicate that at least 2 introductions have occurred. This situation seems to be distinct from that of the Balkans region, where only 1 virus was introduced from Asia and the virus causing human cases has remained genetically stable for decades (11). In fact, circulation of different variants of 1 virus in a small region where it is hyperendemic (Figure 1) in Spain in different years has been described (10), showing that the variability of CCHFV in Spain deserves special attention and efforts to get more sequence information.
Because of the high pathogenicity of CCHFV, a detailed medical history of the patient, including travel history and possible risk factors, is crucial for prompt diagnosis to ensure that appropriate infection control measures can be implemented in a timely manner. For the patient that we report, lack of immediate information regarding the tick bite in combination with the nonspecific initial symptoms meant that CCHF was not suspected until day 8 of illness. The public health services performed contact tracing to identify all persons exposed, and none contracted symptomatic CCHF. This case and the description of a new virus do not modify the risk for infection by CCHFV in Spain. Risk is still considered low, although clinicians at hospitals and general practitioners need to be alert to the possibility of new cases.
Dr. Negredo is a senior researcher at the Arbovirus and Imported Viral Diseases Laboratory of the National Centre for Microbiology (Madrid, Spain). Her primary research interests are viral hemorrhagic fevers and detection of emerging viruses that circulate in Spain.
Acknowledgments
We thank Amir Gacem for reviewing the grammar. We thank Roger Hewson and staff from Public Health England for their support with confirmation and isolation of the virus.
This study was partially funded by ISCIII, RD16CIII/0003/0003, “Red de Enfermedades Tropicales,” Subprogram RETICS Plan Estatal de I+D+I 2013-2016, and co-funded by FEDER “Una manera de hacer Europa.”
References
- Atkinson B, Chamberlain J, Logue CH, Cook N, Bruce C, Dowall SD, et al. Development of a real-time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector Borne Zoonotic Dis. 2012;12:786–93. DOIPubMedGoogle Scholar
- Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, et al.; Crimean Congo Hemorrhagic Fever@Madrid Working Group. Autochthonous Crimean-Congo Hemorrhagic Fever in Spain. N Engl J Med. 2017;377:154–61. DOIPubMedGoogle Scholar
- Ramírez de Arellano E, Hernández L, Goyanes MJ, Arsuaga M, Cruz AF, Negredo A, et al. Phylogenetic characterization of Crimean-Congo hemorrhagic fever virus, Spain. Emerg Infect Dis. 2017;23:2078–80. DOIPubMedGoogle Scholar
- Carroll SA, Bird BH, Rollin PE, Nichol ST. Ancient common ancestry of Crimean-Congo hemorrhagic fever virus. Mol Phylogenet Evol. 2010;55:1103–10. DOIPubMedGoogle Scholar
- Chamberlain J, Cook N, Lloyd G, Mioulet V, Tolley H, Hewson R. Co-evolutionary patterns of variation in small and large RNA segments of Crimean-Congo hemorrhagic fever virus. J Gen Virol. 2005;86:3337–41. DOIPubMedGoogle Scholar
- Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–14. DOIPubMedGoogle Scholar
- Gobierno de España, Ministerio de Sanidad, Consumo y Bienestar Social. Evaluación rápida. Informe de situación y evaluación del riesgo de transmisión del virus de Fiebre Hemorrágica de Crimea-Congo (FHCC) en España [cited 2019 Jul 12]. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/analisisituacion/doc/ER_FHCC.pdf
- Cevik MA, Erbay A, Bodur H, Eren SS, Akinci E, Sener K, et al. Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin Infect Dis. 2007;45:e96–100. DOIPubMedGoogle Scholar
- Cajimat MNB, Rodriguez SE, Schuster IUE, Swetnam DM, Ksiazek TG, Habela MA, et al. Genomic characterization of Crimean-Congo hemorrhagic fever virus in Hyalomma tick from Spain, 2014. Vector Borne Zoonotic Dis. 2017;17:714–9. DOIPubMedGoogle Scholar
- Negredo A, Habela MA, Ramírez de Arellano E, Diez F, Lasala F, López P, et al. Survey of Crimean-Congo hemorrhagic fever enzootic focus in Spain, 2011–2015. Emerg Infect Dis. 2019;25:1177–84. DOIPubMedGoogle Scholar
- Lukashev AN, Klimentov AS, Smirnova SE, Dzagurova TK, Drexler JF, Gmyl AP. Phylogeography of Crimean Congo hemorrhagic fever virus. PLoS One. 2016;11:
e0166744 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 09, 2021
Table of Contents – Volume 27, Number 4—April 2021
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for corresponding Ana Negredo, National Center of Microbiology, Arbovirus and Imported Viral Diseases Laboratory, Crta. Majadahonda–Pozuelo Km 2, 28220-Majadahonda, Madrid, Spain
Top