Volume 27, Number 7—July 2021
Research
Seroprevalence of SARS-CoV-2 among Blood Donors and Changes after Introduction of Public Health and Social Measures, London, UK
Figure 2
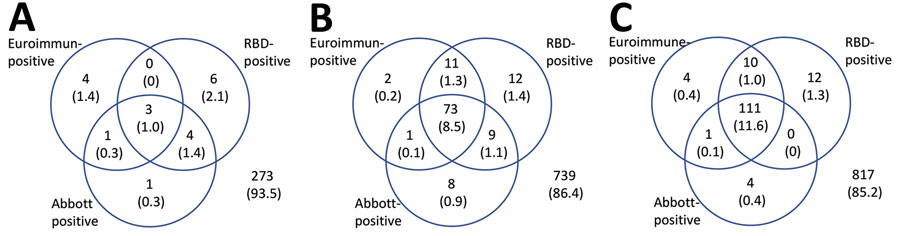
Figure 2. Results for serum samples tested for severe acute respiratory syndrome coronavirus 2 Ig with all 3 assays, by epidemiologic week of sample collection (weeks 13,15–16,and 18), London, UK, 2020. Values are no. (%) of reactive test results. RBD, receptor-binding domain.
Page created: April 19, 2021
Page updated: June 16, 2021
Page reviewed: June 16, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.