Volume 27, Number 8—August 2021
Dispatch
Delayed Antibody and T-Cell Response to BNT162b2 Vaccination in the Elderly, Germany
Figure
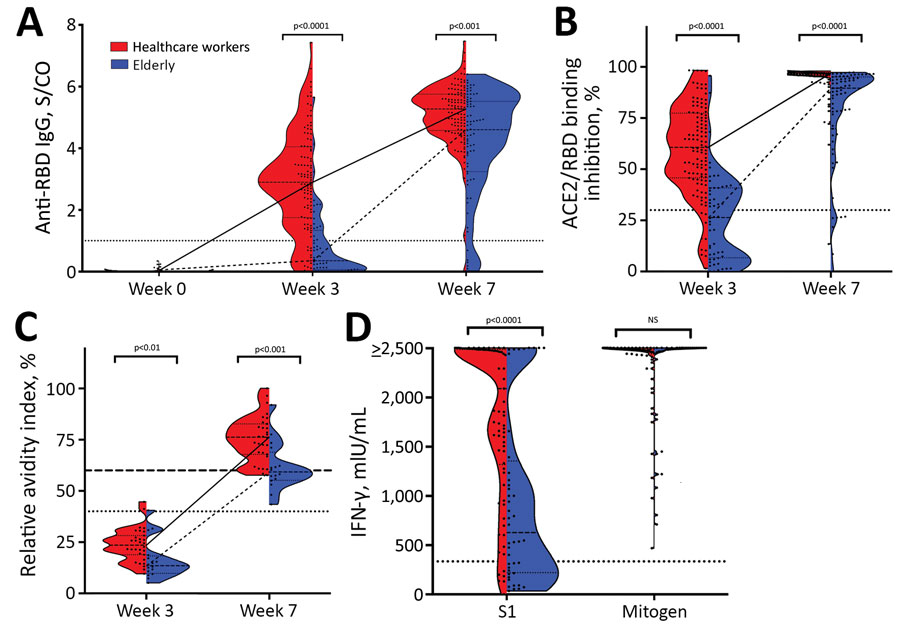
Figure. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibody and T-cell response after vaccination with BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com) in the elderly, Germany. A) SARS-CoV-2 RBD IgG measured in serum of BNT162b2-vaccinated younger participants (healthcare workers) before the first vaccination (n = 100, week 0), 3 weeks after the first vaccination (n = 107, week 3), and 4 weeks after the second vaccination (n = 113, week 7) and from elderly participants at week 0 (n = 70), week 3 (n = 52), and week 7 (n = 70) using the SeraSpot Anti-SARS-CoV-2 IgG assay (Seramun Diagnostica GmbH, https://www.seramun.com). B) Neutralizing capacity of antibodies measured at week 3 and 7 in the young and elderly cohorts using the ELISA-based surrogate virus neutralization test (sVNT) cPass (medac GmbH, https://international.medac.de). C) SARS-CoV-2 spike IgG avidity analyzed in the healthcare workers cohort (n = 30) and elderly cohort (n = 16) at week 3 and 7. D) At week 7, whole blood from vaccinated elderly participants (n = 43) and young participants (n = 71) was stimulated ex vivo with components of the S1 domain of the spike protein for 24 h, and IFN-γ concentration in the supernatant was detected by ELISA. Dotted lines indicate the manufacturer’s specified threshold for RBD IgG >1 S/Co, for sVNT >30%, and for avidity 40–60% borderline avidity and >60% high avidity. For IGRA, we defined an arbitrary threshold at 334.2 mIU/mL. p value was calculated by the nonparametric Mann Whitney U test, and the median and interquartile range are depicted. ACE2, angiotensin-converting enzyme 2; IFN-γ, interferon-γ; IU, international units; NS, not significant; RBD, receptor-binding domain; S/CO, signal-to-cutoff ratio; sVNT, surrogate virus neutralization test.
1These authors contributed equally to this article.
2These senior authors contributed equally to this article.