Volume 27, Number 9—September 2021
Dispatch
Ecologic Determinants of West Nile Virus Seroprevalence among Equids, Brazil
Figure 2
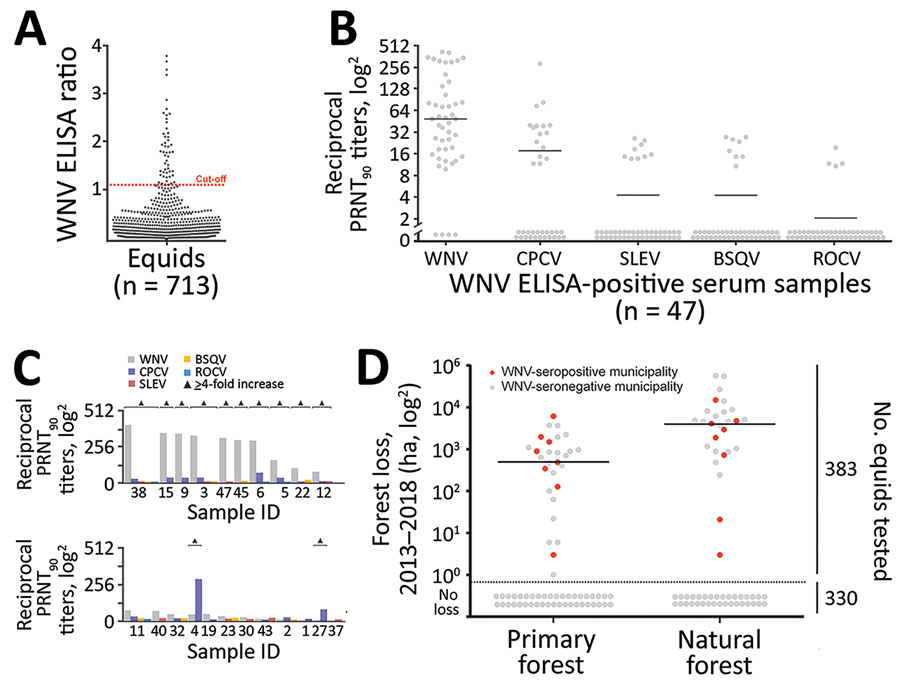
Figure 2. WNV seroprevalence among equids, Brazil. A) ELISA absorbance values displayed as sample to cutoff ratio, as previously described (2). We increased the ELISA cutoff by 10% above which samples were considered positive to maximize specificity because the ELISA was not originally validated for horses in Latin America, which are infected by more Japanese encephalitis serocomplex viruses compared with horses in Europe. Dotted orange line represents the 1.1 positivity cutoff. B) Reciprocal PRNT90 titers for WNV and other flaviviruses. Statistical significance levels were inferred by using the Kruskal-Wallis test. Bars indicate mean. Graph created by using Prism (GraphPad software, https://www.graphpad.com). C) Distinction of heterotypic serum samples based on the endpoint titers of various flaviviruses. Triangles indicated endpoint titers >4-fold. D) Effects of forests and forest loss on WNV seropositivity and seronegativity among equids in municipalities, Brazil. Natural forest is made up of introduced or native tree or vegetation that have reproduced naturally, without help or (human) intervention. Primary forest is made up of intact and nonintact natural forest and refers to areas that reached the final stage of succession during 2013–2018. Data on primary and natural forest were retrieved from Global Forest Watch (http://www.globalforestwatch.org). Right y-axis represents number of total number of equids tested for seroprevalence. Horizontal bars indicate means. Areas below dotted line had no forest loss. BSQV, Bussuquara virus; CPCV, Cacipacoré virus; ha, hectare (10,000 m2); PRNT90, 90% plaque-reduction neutralization test; ROCV, Rocio virus; SLEV, Saint Louis encephalitis virus; WNV, West Nile virus.
References
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308–15. DOIPubMedGoogle Scholar
- Rockstroh A, Moges B, Berneck BS, Sattler T, Revilla-Fernández S, Schmoll F, et al. Specific detection and differentiation of tick-borne encephalitis and West Nile virus induced IgG antibodies in humans and horses. Transbound Emerg Dis. 2019;66:1701–8. DOIPubMedGoogle Scholar
- Dodd RY, Foster GA, Stramer SL. Keeping blood transfusion safe from West Nile virus: American Red Cross experience, 2003 to 2012. Transfus Med Rev. 2015;29:153–61. DOIPubMedGoogle Scholar
- Ward MP, Scheurmann JA. The relationship between equine and human West Nile virus disease occurrence. Vet Microbiol. 2008;129:378–83. DOIPubMedGoogle Scholar
- Mattar S, Edwards E, Laguado J, González M, Alvarez J, Komar N. West Nile virus antibodies in Colombian horses. Emerg Infect Dis. 2005;11:1497–8. DOIPubMedGoogle Scholar
- Morales MA, Barrandeguy M, Fabbri C, Garcia JB, Vissani A, Trono K, et al. West Nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis. 2006;12:1559–61. DOIPubMedGoogle Scholar
- Silva JR, Medeiros LC, Reis VP, Chavez JH, Munhoz TD, Borges GP, et al. Serologic survey of West Nile virus in horses from Central-West, Northeast and Southeast Brazil. Mem Inst Oswaldo Cruz. 2013;108:921–3. DOIPubMedGoogle Scholar
- Ometto T, Durigon EL, de Araujo J, Aprelon R, de Aguiar DM, Cavalcante GT, et al. West Nile virus surveillance, Brazil, 2008-2010. Trans R Soc Trop Med Hyg. 2013;107:723–30. DOIPubMedGoogle Scholar
- Vieira MACS, Romano APM, Borba AS, Silva EVP, Chiang JO, Eulálio KD, et al. West Nile virus encephalitis: the first human case recorded in Brazil. Am J Trop Med Hyg. 2015;93:377–9. DOIPubMedGoogle Scholar
- Martins LC, Silva EVPD, Casseb LMN, Silva SPD, Cruz ACR, Pantoja JAS, et al. First isolation of West Nile virus in Brazil. Mem Inst Oswaldo Cruz. 2019;114:
e180332 . DOIPubMedGoogle Scholar - Fischer C, de Oliveira-Filho EF, Drexler JF. Viral emergence and immune interplay in flavivirus vaccines. Lancet Infect Dis. 2020;20:15–7. DOIPubMedGoogle Scholar
- Pauvolid-Corrêa A, Campos Z, Juliano R, Velez J, Nogueira RM, Komar N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS Negl Trop Dis. 2014;8:
e2706 . DOIPubMedGoogle Scholar - Moreira-Soto A, de Souza Sampaio G, Pedroso C, Postigo-Hidalgo I, Berneck BS, Ulbert S, et al. Rapid decline of Zika virus NS1 antigen-specific antibody responses, northeastern Brazil. Virus Genes. 2020;56:632–7. DOIPubMedGoogle Scholar
- Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584:398–402. DOIPubMedGoogle Scholar
- Swaddle JP, Calos SE. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PLoS One. 2008;3:
e2488 . DOIPubMedGoogle Scholar
1These first authors contributed equally to this article.