Volume 27, Number 9—September 2021
Research Letter
Invasive Meningococcal Disease, 2011–2020, and Impact of the COVID-19 Pandemic, England
Cite This Article
Citation for Media
Abstract
Invasive meningococcal disease incidence in England declined from 1.93/100,000 persons (1,016 cases) in 2010–11 to 0.95/100,000 (530 cases) in 2018–19 and 0.74/100,000 in 2019–20 (419 cases). During national lockdown for the coronavirus disease pandemic (April–August 2020), incidence was 75% lower than during April–August 2019.
Neisseria meningitidis is a major global cause of bacterial meningitis and septicemia (1). Six serogroups (A, B, C, W, X, Y) are responsible for most invasive meningococcal disease (IMD) cases (1). In the United Kingdom, implementation of serogroup C (MenC) meningococcal conjugate vaccine in 1999 led to sustained declines in MenC disease (2). In August 2015, an emergency adolescent MenACWY immunization program for persons 13–18 years of age and new university students was implemented to control a national outbreak of a hypervirulent MenW strain belonging to sequence type 11 clonal complex (MenW:cc11) (3). In September 2015, the United Kingdom became the first country to add a protein-based meningococcal B vaccine, 4CMenB, into the national infant immunization program (4). Both programs have reduced IMD caused by the respective vaccine serogroups (5).
Since December 2019, the novel coronavirus (COVID-19) pandemic has led to major changes in the epidemiology of bacterial and viral infections worldwide (Brueggemann AB et al., unpub. data, https://www.medrxiv.org/content/10.1101/2020.11.18.20225029v1). We report IMD incidence in England during 2011–2020, including the impact of a national lockdown to control the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
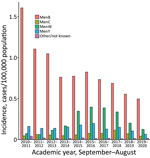
Public Health England (PHE) conducts national surveillance of IMD (6) and SARS-CoV-2 (7) in England. IMD incidence was highest, 1.93 cases/100,000 population (1,016 total cases), during the 2010–11 academic year (September–August) and declined to 1.15 cases /100,000 population for 2013–14 (617 cases) before increasing to 1.51 cases /100,000 population (825 cases) in 2015–16 (Figure). Adolescent MenACWY and infant 4CMenB immunization programs in 2015 led to additional annual declines in IMD incidence, to 0.95 cases /100,000 population (530 cases) in 2018–19 (incidence rate ratio [IRR] 0.63 [95% CI 0.56–0.70] for 2018–19 vs. 2015–16 ). Incidence further declined during the 2019–20 pandemic year (419 cases; 0.74 cases /100,000 population; IRR 0.49 [95% CI 0.44–0.56] for 2019–20 vs. 2015–16). IMD cases declined for all serogroups from 2015–16 to 2019–20: MenB by 38% (from 452 to 279 cases), MenC by 41% (41 to 24 cases), MenW by 68% (218 to 70 cases) and MenY by 66% (108 to 37 cases).
IMD cases declined after the national COVID-19 lockdown on March 23, 2020, and remained low during April–August 2020. During 2018–19, PHE received 12,628 clinical samples from patients with suspected IMD; of these, 462 (4%) tested positive for N. meningitidis. These totals were 9,968 specimens, 401 (4%) positive, during 2019–20 (21% fewer cases). During April–August 2020, a total of 50 (1.8%) of 2,808 samples tested positive for N. meningitidis, compared with 134 (2.7%) of 5,025 samples during the same period in 2019 (p = 0.016). Combining culture-confirmed and PCR-confirmed cases, IMD incidence was 75% lower (IRR 0.25, 95% CI 0.18–0.35) during April–August 2020 than during April–August 2019 (Table). In contrast, IMD incidence during September 2019–March 2020 (the 7 months before national lockdown) was similar to that for September 2018–March 2019 (IRR 1.06, 95% CI 0.91–1.23). Declines were observed for all age groups and serogroups. During lockdown, compared with the same period during the previous year, MenB was overrepresented (33/45 [73%] vs. 104/179 [58%] cases), whereasMenW (5/45 [11%] vs. 42/179 [23%] cases) and MenY (0/45 [0%] vs. 16/179 [9%] cases) were underrepresented.
A total of 45 IMD cases were diagnosed during April–August 2020. The median age of patients was 67 (interquartile range 20–85) years. Linkage with national SARS-CoV-2 data identified 2 patients with IMD who were also positive for SARS-CoV-2 by reverse transcription PCR; both were <90 days of age with late-onset MenB meningitis, and 1 died. Meningitis (with or without septicemia) was proportionally more frequent during the lockdown months compared with the same period in 2019 (27/45 [60%] v. 71/179 [39.7%] cases; p = 0.014). Three (6.7%) of the 45 patients died within 28 days of diagnosis: the infant with co-infection, an adult with MenB meningitis, and an older adult with MenB septicemia.
Limitations of our study include limited clinical data collected for IMD cases. Cases and case-fatality rates during the lockdown period might also be underestimated if some patients died of IMD at home because they did not seek medical help earlier as a result of the stay at home messaging during lockdown.
In summary, IMD incidence in England has been declining since the early 2000s (8) because of the MenC immunization program and natural trends in MenB disease and further declined because of 2 new meningococcal immunization programs. National lockdown in March 2020 led to a 75% reduction in cases compared with the same period in the previous year, overrepresented MenB cases. Declines in IMD cases after national lockdown were also reported in France (9), which is reassuring because viral infections are known to precede IMD; therefore, SARS-CoV-2 could potentially have increased the risk of secondary bacterial infections. Our findings do not support wider vaccination against IMD during the COVID-19 pandemic.
Dr. Subbarao is a microbiology and infection diseases registrar for Public Health England in London. Her main interests are vaccine-preventable diseases, antimicrobial stewardship, and M. tuberculosis.
References
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. DOIPubMedGoogle Scholar
- Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–6. DOIPubMedGoogle Scholar
- Campbell H, Edelstein M, Andrews N, Borrow R, Ramsay M, Ladhani S. Emergency meningococcal ACWY Vaccination program for teenagers to control group W meningococcal eisease, England, 2015–2016. Emerg Infect Dis. 2017;23:1184–7. DOIPubMedGoogle Scholar
- Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382:309–17. DOIPubMedGoogle Scholar
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388:2775–82. DOIPubMedGoogle Scholar
- Ladhani SN, Waight PA, Ribeiro S, Ramsay ME. Invasive meningococcal disease in England: assessing disease burden through linkage of multiple national data sources. BMC Infect Dis. 2015;15:551. DOIPubMedGoogle Scholar
- Public Health England. Research and analysis: national COVID-19 surveillance reports: GOV.UK; 2021 [cited 2020 Oct 2]. https://www.gov.uk/government/publications/national-covid-19-surveillance-reports
- Ladhani SN, Flood JS, Ramsay ME, Campbell H, Gray SJ, Kaczmarski EB, et al. Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine. 2012;30:3710–6. DOIPubMedGoogle Scholar
- Taha MK, Deghmane AE. Impact of COVID-19 pandemic and the lockdown on invasive meningococcal disease. BMC Res Notes. 2020;13:399. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: June 30, 2021
Table of Contents – Volume 27, Number 9—September 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Addresses for correspondence: Shamez Ladhani or Sathyavani Subbarao, Immunisation and Countermeasures Division, Public Health England, 61 Colindale Ave, London NW9 5EQ, UK
Top