Volume 28, Number 12—December 2022
Dispatch
Sylvatic Transmission of Chikungunya Virus among Nonhuman Primates in Myanmar
Abstract
Nonhuman primates living in proximity to humans increase risks for sylvatic arbovirus transmission. We collected serum samples from nonhuman primates in Hlawga National Park near Yangon, Myanmar, and detected antibodies against chikungunya (33%) and Japanese encephalitis (4%) viruses. Buffer zones between primate and human communities might reduce cross-species arbovirus transmission.
Several endemic and emerging arboviruses, such as chikungunya (CHIKV), Zika (ZIKV), and dengue (DENV) viruses, have evolutionary origins in nonhuman primates (NHPs) (1,2). These pathogens have adapted sylvatic to urban transmission cycles by using humans as amplifying hosts where NHPs are no longer required for virus maintenance. However, sylvatic arbovirus transmission cycles involving NHPs could act as sources of human infections, which would affect public health. NHPs could enable reemergence of arbovirus infections after immunity has waned following human–mosquito–human transmission. Sylvatic cycles can also provide selective environments where new viral strains can emerge.
CHIKV circulates in distinct enzootic, sylvatic transmission cycles in old world monkeys in the forests of sub-Saharan Africa (2). Limited data are available on sylvatic CHIKV transmission in Asia, but seroconversion has been detected in cynomolgus macaques (Macaca fascicularis), pig-tailed macaques (M. nemistrina), black-crested Sumatran langurs (Presbytis melalophos), and dusky leaf monkeys (Presbytis obscura) in Thailand (3,4), and virus has been isolated from long-tailed macaques in Malaysia (5). A sylvatic ZIKV lineage in Africa, infecting Cercopthecidae primate species, is known to circulate widely (6). The only positive ZIKV serology findings in primates in Asia have been in orangutans (Pongo pygmaeus) in Borneo, Malaysia, but those exposures were likely from an urban strain (7). Sylvatic DENV cycles occur in the forests of Malaysia in Macaca and Presbytis spp. monkeys (8) and also in West Africa; sylvatic DENV-2 circulates regularly between Erythrocebus patas monkeys and various Aedes spp. mosquitoes in Senegal (8). Seroprevalence of Japanese encephalitis virus (JEV) has been reported in cynomolgus monkeys, Japanese macaques (M. fuscata), green monkeys (Chlorocebus sabaeus), and pig-tailed macaques in several countries in Asia (4,9,10).
Myanmar is among the least studied but most heavily forested region in Asia, and CHIKV, ZIKV, DENV and JEV are highly endemic in humans. We investigated whether Myanmar peri-urban primates, living near the largest urban city of Yangon, are exposed to arboviruses of public health concern and could be sources of spillover or recipients of spillback of human pathogenic arboviral diseases.
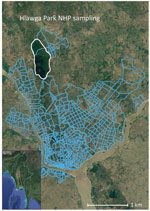
We collected specimens from 107 rhesus monkeys (Macaca mulata) and 12 pig-tailed macaques within Hlawga National Park, an open zoo and wildlife sanctuary in Myanmar’s Yangon region that covers 6.23 km2 (Figure). NHPs are free ranging within this park and have frequent opportunities for human contact. Serum samples were collected during October 2016–August 2017, which spanned 2 dry/wet seasons. We used a Luminex xMAP multiplex bead-based assay (Luminex Corp., https://www.luminexcorp.com) to simultaneously measure total IgG, IgA, and IgM against CHIKV E1 envelope protein, ZIKV nonstructural protein 1 (NS1), ZIKV envelope protein, DENV-1–4 NS1, JEV NS1, West Nile virus NS1, yellow fever virus NS1, and tickborne encephalitis virus NS1 (Appendix Table). We confirmed positive serum samples by using the plaque reduction neutralization test (Appendix). Conventional reverse transcription PCR targeting conserved regions of Flavivirus and Alphavirus spp. was performed to detect arbovirus viremia (Appendix).
We identified virus-reactive antibodies among NHPs in Hlawga National Park, suggesting prior exposure to arboviruses, but we did not detect viruses by using PCR, suggesting absence of active infections. We found 33% (39/119) of NHPs were seropositive for CHIKV and 4% (5/119) were seropositive for JEV (Table); all serum samples were negative for ZIKV, West Nile virus, yellow fever virus, and tick-borne encephalitis virus. Using bivariate analysis, we showed specimens collected during the dry season were more likely to be seropositive for CHIKV (p = 0.05). Greater proportions of adult NHPs appeared to be seropositive for CHIKV; however, the difference was not statistically significant. We found no statistically significant associations between sex, age class, or species and specific arbovirus exposure. CHIKV and JEV in NHPs in Myanmar have not been reported, likely because of limited surveillance. Our findings extend the geographic range of potential sylvatic cycles for CHIKV to forests and peri-urban areas of Myanmar.
Our results indicate that NHPs were exposed to CHIKV during a period with no or limited human–mosquito–human transmission, suggesting that seropositive samples resulted from sylvatic exposures. IgG against CHIKV E2 protein can be detected up to 21 months postinfection (11). If similar kinetics occur in NHPs and extend to the E1 protein, NHP exposures to CHIKV could have occurred during 2013–2014 or earlier. However, in 2017, we detected CHIKV antibodies in NHPs that were <5 years of age, indicating exposure during an interepidemic period. Human cases of CHIKV were not reported by the Myanmar Ministry of Health during 2011–2018 (12), and CHIKV outbreaks are not commonly underreported because a large proportion of infected persons have indicative arthritic manifestations. In 2019, health officials reported widespread outbreaks of CHIKV in Mandalay, Nay Pyi Taw, Kachin State, Tanintharyi, and Yangon regions of Myanmar, indicating reemergence of the virus (12).
We studied an NHP population that lived in a forested area outside of Yangon and could have played a role in the reemergence of CHIKV in humans. The large proportion of NHPs that were exposed indicated the virus was circulating among sylvatic mosquitos and primates in this park. The absence of reported human infections during the potential period of NHP infection suggested that spillover from humans to NHPs via mosquitoes was unlikely. Aedes aegypti and A. albopictus mosquitoes, the two primary urban vectors of CHIKV, are also known to feed almost exclusively on humans in the region, providing further evidence that NHP exposures to CHIKV in our study population were of sylvatic origin (13).
Our findings indicate that JEV is circulating at the periphery of Yangon, and NHPs can be occasional incidental hosts. JEV is endemic in Myanmar, particularly in the Yangon region (14). NHPs are not thought to be potential reservoirs, but are dead-end hosts; they produce a low viremia that cannot subsequently infect mosquitoes (1). Low levels of viremia produced in experimental studies and sylvatic cycles involving waterfowl or pigs are well documented. Furthermore, JEV is transmitted to humans by infected Culex spp. mosquitoes (most commonly Culex tritaeniorhynchus), which feed on many mammals in the region (15), making it more plausible that NHPs could be incidental targets of this mosquito species.
We did not confirm NHP exposure to DENV or ZIKV. We identified positive samples by using the Luminex assay, but those samples tested negative when the plaque reduction neutralization assay was used for confirmation. DENV is endemic in humans in Myanmar, and our findings indicate that spillback of urban DENV strains to NHPs is not common in this region or was not detected in our sample size. Given the limited knowledge of the scope of human ZIKV circulation in Myanmar and lack of entomological data, further research is needed to examine potential sylvatic ZIKV cycles among NHPs in Asia.
Our study demonstrates the importance of conducting surveillance of peri-urban primates in regions of high arbovirus transmission and the need for less invasive methods that improve feasibility. Future research on molecular epidemiology of arboviruses in humans, NHPs, and mosquitoes is needed to confirm whether exposures result from potential sylvatic cycles of ongoing transmission or spillback events from urban strains. A heightened awareness of new CHIKV outbreak potential in humans living near NHPs in Hlawga National Park is warranted. Buffer zones between parks and human settlements might reduce future cross-species arbovirus transmission.
Dr. Smiley Evans is a research epidemiologist at the One Health Institute, University of California, Davis, CA, USA. Her research focuses on disease transmission dynamics between humans and wildlife and the effects of biodiversity on disease emergence.
Acknowledgments
We thank the Livestock Breeding and Veterinary Department, the Forest Department, and the Department of Medical Research of the Republic of the Union of Myanmar for their support of this research. We thank Than Toe, Than Swe, Khin Maung Win, and Aung Than Toe for their guidance on working in Myanmar.
This research was supported by the Fogarty International Center of the National Institutes of Health (grant no. K01TW010279), the National Institute of Allergy and Infectious Diseases (grant no. 1U01AI151814-01), and the US Agency for International Development (USAID) Emerging Pandemic Threats PREDICT project (cooperative agreement no. GHN-A-OO-09-00010-00).
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the National Institutes of Health or US Agency for International Development.
References
- Weaver SC, Barrett ADT. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2:789–801. DOIPubMedGoogle Scholar
- Weaver SC, Winegar R, Manger ID, Forrester NL. Alphaviruses: population genetics and determinants of emergence. Antiviral Res. 2012;94:242–57. DOIPubMedGoogle Scholar
- Marchette NJ, Rudnick A, Garcia R, MacVean DW. Alphaviruses in Peninusular Malaysia: I. Virus isolations and animal serology. Southeast Asian J Trop Med Public Health. 1978;9:317–29.PubMedGoogle Scholar
- Nakgoi K, Nitatpattana N, Wajjwalku W, Pongsopawijit P, Kaewchot S, Yoksan S, et al. Dengue, Japanese encephalitis and Chikungunya virus antibody prevalence among captive monkey (Macaca nemestrina) colonies of Northern Thailand. Am J Primatol. 2014;76:97–102. DOIPubMedGoogle Scholar
- Apandi Y, Nazni WA, Azleen ZAN, Vythilingham I, Noorazian MY, Azahari AH, et al. The first isolation of chikungunya virus from nonhuman primates in Malaysia. J Gen Mol Virol. 2009;1:035–9.
- Faria NR, Azevedo RDSDS, Kraemer MUG, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–9. DOIPubMedGoogle Scholar
- Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64:310–6. DOIPubMedGoogle Scholar
- Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9:532–41. DOIPubMedGoogle Scholar
- Yuwono J, Suharyono W, Koiman I, Tsuchiya Y, Tagaya I. Seroepidemiological survey on dengue and Japanese encephalitis virus infections in Asian monkeys. Southeast Asian J Trop Med Public Health. 1984;15:194–200.PubMedGoogle Scholar
- Inoue S, Morita K, Matias RR, Tuplano JV, Resuello RRG, Candelario JR, et al. Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines. J Med Primatol. 2003;32:89–94. DOIPubMedGoogle Scholar
- Kam YW, Lee WWL, Simarmata D, Harjanto S, Teng TS, Tolou H, et al. Longitudinal analysis of the human antibody response to Chikungunya virus infection: implications for serodiagnosis and vaccine development. J Virol. 2012;86:13005–15. DOIPubMedGoogle Scholar
- Luvai EAC, Kyaw AK, Sabin NS, Yu F, Hmone SW, Thant KZ, et al. Evidence of Chikungunya virus seroprevalence in Myanmar among dengue-suspected patients and healthy volunteers in 2013, 2015, and 2018. PLoS Negl Trop Dis. 2021;15:
e0009961 . DOIPubMedGoogle Scholar - Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–9. DOIPubMedGoogle Scholar
- Thaung Y, Swaddiwudhipong W, Tin H, Thammawijaya P, Thitichai P, Tin TC. Epidemiological features of Japanese encephalitis among acute encephalitis syndrome cases in Myanmar, 2014–2016: implications to the vaccination program. OSIR. 2019;12:24–31. http://www.osirjournal.net/index.php/osir/article/view/137
- Reuben R, Thenmozhi V, Samuel PP, Gajanana A, Mani TR. Mosquito blood feeding patterns as a factor in the epidemiology of Japanese encephalitis in southern India. Am J Trop Med Hyg. 1992;46:654–63. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: November 18, 2022
Table of Contents – Volume 28, Number 12—December 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tierra Smiley Evans, One Health Institute, University of California, Davis, 1089 Veterinary Medicine Dr, Veterinary Medicine 3B, Davis, CA 95616, USA;tsmevans@ucdavis.edu
Top