Volume 28, Number 3—March 2022
Research
Effectiveness of 3 COVID-19 Vaccines in Preventing SARS-CoV-2 Infections, January–May 2021, Aragon, Spain
Figure 2
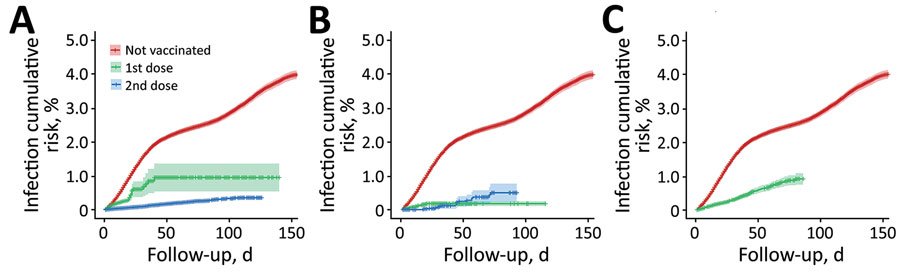
Figure 2. Cumulative risk curves (1 minus the Kaplan-Meier risk) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection for 3 coronavirus disease vaccines, Aragon, Spain, January–May 2021. A) BioNTech-Pfizer BNT162b2 mRNA, B) Moderna mRNA-1273, and C) Oxford-AstraZeneca ChAdOx1-S-AZD1222. Shadows across lines represent 95% CI. For unvaccinated participants, 95% CI at day 90 of follow-up was 2.6%–2.8%. For participants who went on to receive the BioNTech-Pfizer vaccine, 95% CI at day 90 of follow-up was 0.5%–1.4% (1 dose) and 0.3%–0.4% (2 doses). For the Moderna vaccine, 95% CI at day 90 of follow-up was 0.1%–0.2% (1 dose), and 0.2%–0.8% (2 doses). For Oxford-AstraZeneca, 95% CI at day 90 of follow-up was 0.7%–1.0% (1 dose). Cumulative risk curves of SARS-CoV-2 infection start from the day after vaccination when full protection against SARS-CoV-2 infection is thought to begin, according to previous studies (1–3). The hairs on both sides of the lines represent participants lost to follow-up; gaps represent periods of time between losses.
References
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al.; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. DOIPubMedGoogle Scholar
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–15. DOIPubMedGoogle Scholar
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al.; Oxford COVID Vaccine Trial Group. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. [Erratum in: Lancet. 2021;397:880]. Lancet. 2021;397:881–91. DOIPubMedGoogle Scholar
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al.; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. DOIPubMedGoogle Scholar
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al.; Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81. DOIPubMedGoogle Scholar
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. DOIPubMedGoogle Scholar
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al.; 2019nCoV-501 Study Group. 2019nCoV-501 Study Group. 2019nCoV-501 Study Group. 2019nCoV-501 Study Group. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1899–909. DOIPubMedGoogle Scholar
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23. DOIPubMedGoogle Scholar
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–29. DOIPubMedGoogle Scholar
- McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. DOIPubMedGoogle Scholar
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–57. DOIPubMedGoogle Scholar
- Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al.; SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–35. DOIPubMedGoogle Scholar
- Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. [Erratum in Lancet Infect Dis. 2021;21:e208]. Lancet Infect Dis. 2021;21:1539–48. DOIPubMedGoogle Scholar
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. DOIPubMedGoogle Scholar
- Johns Hopkins University. Coronavirus resource center. [cited 2021 Aug 1] https://coronavirus.jhu.edu/map.html
- Government of Aragon (Spain). Aragon population register. Population pyramids [in Spanish]. [cited 2021 Aug 1] https://www.aragon.es/-/piramides-de-poblacion.-aragon
- Aragon Department of Health. Aragonese COVID-19 epidemiological report [in Spanish], Zaragoza, Spain [cited 2021 Jul 31] https://datacovid.salud.aragon.es/covid
- García-Montero C, Fraile-Martínez O, Bravo C, Torres-Carranza D, Sanchez-Trujillo L, Gómez-Lahoz AM, et al. An updated review of SARS-CoV-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines (Basel). 2021;9:433. DOIPubMedGoogle Scholar
- European Medicines Agency. COVID-19 vaccines authorized: vaccines authorised in the European Union (EU) to prevent COVID-19, following evaluation by the European Medicines Agency (EMA) [cited 2021 Jun 9]. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised
- Interterritorial Board of the Spanish National Health System. COVID-19 vaccination strategy in Spain, 8th update [in Spanish]. 2021 Jun 22 [cited 2021 Aug 1]. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion8_EstrategiaVacunacion.pdf
- Aragon Department of Health. Action plan for COVID-19 vaccination in Aragon, updated 2021 May 3 [in Spanish] [cited 2021 Aug 1]. https://www.aragon.es/documents/20127/1650151/Plan_Operativo_Vacunacion_Covid19_Aragon_20210503.pdf
- Aragon Department of Health. Aragon weekly epidemiologic bulletin, week 20 (2021 May 17–23) [in Spanish] [cited 2021 Aug 1] https://www.aragon.es/documents/20127/1650151/BEsA_202021.pdf
- Aragon Department of Health. Aragon weekly epidemiologic bulletin, week 29 (2021 July 19–25) [in Spanish] [cited 2021 Aug 1] https://www.aragon.es/documents/20127/1650151/BEsA_292021.pdf
- World Health Organization. Public health surveillance for COVID-19: interim guidance. 2020 Dec 16 [cited 2021 Aug 1]. https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020.8
- Spanish Directorate-General of Public Health, Spanish Department of Health. Strategy for early detection, surveillance and control of COVID-19, updated 2021 Jul 23 [in Spanish] [cited 2021 Aug 1] https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf
- Aragon Department of Health. General procedure for COVID-19 healthcare in Aragon, updated 2021 Jun 29 [in Spanish] [cited 2021 Aug 1]. https://www.aragon.es/documents/20127/1650151/20210629_Procedimiento_COVID_19_Aragon.pdf
- Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, et al.; Control Room Working Group. BNT162b2 mRNA Covid-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021;224:431–4. DOIPubMedGoogle Scholar
- Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. DOIPubMedGoogle Scholar
- Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021;
ciab438 ; Epub ahead of print. DOIPubMedGoogle Scholar - Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, et al.; Working Group for the Study of COVID-19 in Navarra. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill. 2021;26:
2100438 . DOIPubMedGoogle Scholar - Frederiksen LSF, Zhang Y, Foged C, Thakur A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol. 2020;11:1817. DOIPubMedGoogle Scholar
- Randolph HE, Barreiro LB. Herd Immunity: Understanding COVID-19. Immunity. 2020;52:737–41. DOIPubMedGoogle Scholar