Volume 28, Number 4—April 2022
Research Letter
Hantavirus Pulmonary Syndrome in a COVID-19 Patient, Argentina, 2020
Cite This Article
Citation for Media
Abstract
We describe a patient in Argentina with severe acute respiratory syndrome coronavirus 2 infection and hantavirus pulmonary syndrome (HPS). Although both coronavirus disease and HPS can be fatal when not diagnosed and treated promptly, HPS is much more lethal. This case report may contribute to improved detection of co-infections in HPS-endemic regions.
The current coronavirus disease (COVID‐19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has resulted in substantial illness and death rates worldwide. Orthohantaviruses are zoonotic viruses responsible for another severe respiratory infectious disease in the Americas, hantavirus pulmonary syndrome (HPS). Although humans generally become infected with HPS through inhaling excreta generated by infected rodents, person-to-person transmission has been well documented in Argentina and Chile (1–3). Humans become infected with SARS‐CoV‐2 and orthohantaviruses in similar ways, through inhaling contaminated aerosols, and can have onset of similar respiratory syndromes. Despite these similarities, the incubation period is shorter in COVID-19 patients (2–14 days) than in HPS patients (7–45 days). Furthermore, at the time the case we describe was reported, the cumulative case-fatality rate for COVID-19 in Argentina was 2.7% (4); for HPS, it was 22%–40% (5).
HPS is characterized by the onset of symptoms such as fever, myalgia, cough, dyspnea, diarrhea, and sweating. Rapid progression to shock or respiratory distress can occur within hours. Symptom-based therapy with oxygen and ventilatory or circulatory support is needed (6,7).
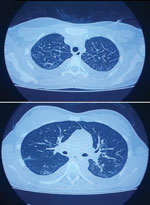
We describe a case of SARS‐CoV‐2 and Andes virus co-infection in central Argentina. The patient, a 22-year-old woman without relevant pathologic records, sought care at a local hospital in November 2020 for fever, headache, myalgia, and gastrointestinal manifestations. A nasopharyngeal swab sample tested positive for SARS-CoV-2 by reverse transcription PCR at the Instituto Biológico “Tomás Perón” (Appendix). Five days after the onset of fever, the patient’s clinical status worsened, and she was admitted to the hospital. Clinical laboratory findings at admission indicated thrombocytopenia, high leukocyte count, lymphopenia, and elevated hepatic enzymes (Appendix). Computed tomography revealed bilateral pleural effusion associated with interstitial infiltration, and capillary filtration with slight peripheral pulmonary ground-glass opacity (Figure; Video).
Within a few hours after admission, the patient had onset of marked respiratory distress. She was then referred to the intensive care unit for orotracheal intubation and treated with ampicillin/sulbactam and azithromycin. The epidemiologic investigation established that the patient resided in a hantavirus-endemic area. Consequently, HPS was suspected, despite the COVID-19–positive diagnosis. According to the confirmation criteria used by the Hantavirus National Reference Laboratory (8), Andes virus infection was confirmed by the detection of specific IgM and IgG by ELISA and genomic viral RNA by quantitative reverse transcription PCR in blood (Appendix).
Three days after the co-infection was confirmed, the patient was extubated and progressed favorably. Twenty days after onset of symptoms, she was discharged from the hospital.
To determine the viral genotype of Andes virus, we conducted a nucleotide sequence analysis from 2 partial fragments of viral small (496-bp) and medium (611-bp) segments, and we submitted the sequences obtained to GenBank (accession nos. OL840325 and OL840326). The highest nucleotide identities matched previous published sequences corresponding to Plata genotype of Andes virus (GenBank accession nos. EU564720 [96% identity] and AY101185 [97.8 identity]). This viral genotype is one of the prevalent pathogenic orthohantaviruses circulating in central Argentina and Uruguay (9).
Because the incubation period for HPS is longer than that for COVID-19, we might speculate that hantavirus infection occurred before coronavirus infection. The respiratory distress syndrome appeared 5 days after the onset of fever, which coincided with the characteristic prodromal period described for HPS. This condition, during the incubation period of HPS, could have induced a higher susceptibility to COVID-19. Because HPS can evolve rapidly to respiratory failure in most patients with severe disease, resulting in high case-fatality rates, alerting health-care workers from HPS-endemic areas is warranted to detect co-infections in the context of the COVID-19 pandemic. In particular, at least 2 genotypes of Andes virus can be transmitted person-to-person, and these species are prevalent in 2 of the 3 hantavirus-endemic regions of Argentina (10).
In conclusion, we detected co-infection with SARS-CoV-2 and Andes virus causing HPS in a patient from a hantavirus-endemic area. Clinicians should be aware of the possibility of co-infection for patients originating, residing, or traveling in hantavirus-endemic areas.
Miss Coelho works in the Virology Department, Instituto Malbran, Buenos Aires, Argentina. Her primary research interests include epidemiology of infectious diseases and the diagnosis of respiratory viruses. Dr. Periolo works in the Virology Department, Instituto Malbran, Buenos Aires, Argentina. Her primary research interests include the virology of infectious diseases, immunology, and infectious respiratory diseases.
References
- Alonso DO, Pérez-Sautu U, Bellomo CM, Prieto K, Iglesias A, Coelho R, et al. Person-to-person transmission of Andes virus in hantavirus pulmonary syndrome, Argentina, 2014. Emerg Infect Dis. 2020;26:756–9. DOIPubMedGoogle Scholar
- Martínez VP, Di Paola N, Alonso DO, Pérez-Sautu U, Bellomo CM, Iglesias AA, et al. “Super-spreaders” and person-to-person transmission of Andes virus in Argentina. N Engl J Med. 2020;383:2230–41. DOIPubMedGoogle Scholar
- Riquelme R, Rioseco ML, Bastidas L, Trincado D, Riquelme M, Loyola H, et al. Hantavirus pulmonary syndrome, Southern Chile, 1995-2012. Emerg Infect Dis. 2015;21:562–8. DOIPubMedGoogle Scholar
- Ministerio de Salud Argentina. Boletín integrado de vigilancia N525 SE49, 11/01/2021 [cited 2021 Jan 21]. https://bancos.salud.gob.ar/sites/default/files/2021-01/biv_525_se49.pdf
- Martinez VP, Bellomo CM, Cacace ML, Suárez P, Bogni L, Padula PJ. Hantavirus pulmonary syndrome in Argentina, 1995-2008. Emerg Infect Dis. 2010;16:1853–60. DOIPubMedGoogle Scholar
- MacNeil A, Ksiazek TG, Rollin PE. Hantavirus pulmonary syndrome, United States, 1993-2009. Emerg Infect Dis. 2011;17:1195–201. DOIPubMedGoogle Scholar
- Jonsson CB, Hooper J, Mertz G. Treatment of hantavirus pulmonary syndrome. Antiviral Res. 2008;78:162–9. DOIPubMedGoogle Scholar
- Alonso DO, Iglesias A, Coelho R, Periolo N, Bruno A, Córdoba MT, et al. Epidemiological description, case-fatality rate, and trends of Hantavirus Pulmonary Syndrome: 9 years of surveillance in Argentina. J Med Virol. 2019;91:1173–81. DOIPubMedGoogle Scholar
- Padula PJ, Colavecchia SB, Martínez VP, Gonzalez Della Valle MO, Edelstein A, Miguel SD, et al. Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. J Clin Microbiol. 2000;38:3029–35. DOIPubMedGoogle Scholar
- Martinez VP, Bellomo C, San Juan J, Pinna D, Forlenza R, Elder M, et al. Person-to-person transmission of Andes virus. Emerg Infect Dis. 2005;11:1848–53. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: February 24, 2022
1These authors contributed equally to this article.
Table of Contents – Volume 28, Number 4—April 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Natalia Periolo, Instituto Nacional de Enfermedades Infecciosas, Administración Nacional de Laboratorios e Institutos de Salud, Av Velez Sarsfield 563, C1282 AFF, CABA, Argentina
Top