Volume 28, Number 4—April 2022
Research Letter
Early Circulation of SARS-CoV-2, Congo, 2020
Figure
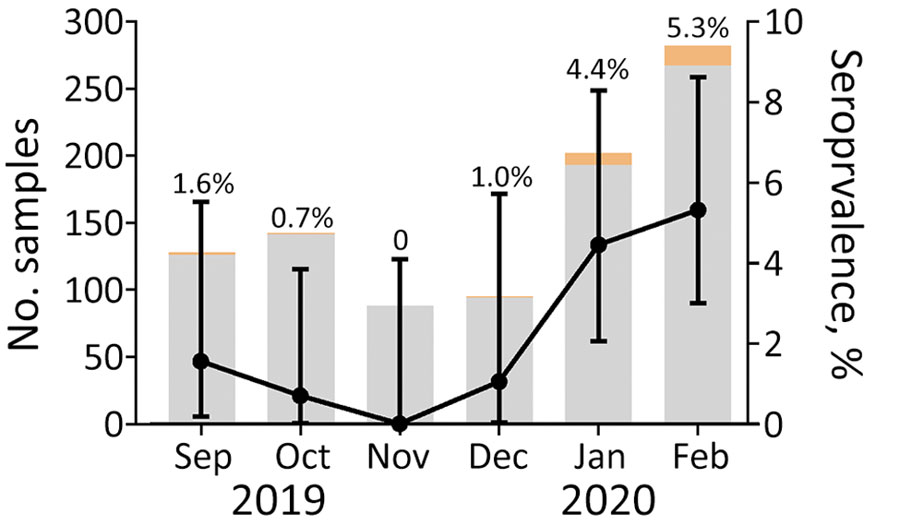
Figure. Number of plasma samples tested each month for severe acute respiratory syndrome coronavirus antibodies by using a microsphere immunoassay with beads coupled with receptor-binding domain antigen, Congo, September 2019–February 2020. Gray shading indicates the number of seronegative samples; orange, seropositive samples. Seropositivity is represented by black dots; error bars indicate 95% binomial CIs.
1These first authors contributed equally to this article.
Page created: February 07, 2022
Page updated: March 19, 2022
Page reviewed: March 19, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.