Volume 28, Number 7—July 2022
Research
Effect of Agroecosystems on Seroprevalence of St. Louis Encephalitis and West Nile Viruses in Birds, La Pampa, Argentina, 2017–2019
Cite This Article
Citation for Media
Abstract
In Argentina, the Pampa ecoregion has been almost completely transformed into agroecosystems. To evaluate the environmental (agricultural area, tree coverage, distance to the nearest water body and urban site) and biological (dove, cowbird, and sparrow abundance) effects on free-ranging bird exposure to St. Louis encephalitis virus (SLEV) and West Nile virus (WNV), we used generalized linear mixed models. For 1,019 birds sampled during 2017–2019, neutralizing antibodies were found against SLEV in samples from 60 (5.8%) birds and against WNV for 21 (2.1%). The best variable for explaining SLEV seroprevalence was agricultural area, which had a positive effect; however, for WNV, no model was conclusive. Our results suggest that agroecosystems in the La Pampa ecoregion increase the exposure of avian hosts to SLEV, thus potentially increasing virus activity.
Zoonotic infections, particularly those transmitted from one vertebrate to another by an arthropod vector (vectorborne diseases), have been frequently identified among the most common emerging infectious diseases (1). During recent decades, reemergence of many such pathogens (e.g., dengue virus, yellow fever virus, Zika virus, chikungunya virus, Saint Louis encephalitis virus [SLEV], and West Nile virus [WNV]) represents a threat to human health and wildlife conservation (2).
SLEV and WNV belong to the family Flaviviridae, genus Flavivirus. SLEV is endemic to the Americas and has recently re-emerged in the western United States (3,4), southern Brazil, and central Argentina (5). In Argentina, according to ecologic studies, the SLEV transmission network is integrated by Culex quinquefasciatus, Culex interfor, and Culex saltanensis mosquitoes as vectors (6) and eared doves (Zenaida auriculata) and Picui ground doves (Columbina picui) as amplifying urban hosts (7). WNV was first detected in the Americas in 1999, causing an encephalitis outbreak among humans and massive mortality events among American crows (Corvus brachyrhynchos) (8). In 2006 in Argentina, the virus was isolated from sick horses in Buenos Aires and Entre Ríos Provinces (9). However, serologic evidence from free-ranging birds indicates previous endemic WNV activity in a large mosaic of resident birds from central and northern Argentina since 2004 (10). Vector competence studies have indicated that Cx. quinquefasciatus and Cx. interfor mosquitoes are able to transmit the local strain of WNV (11), whereas host competence studies have identified the Picui ground dove as an amplifier host for WNV (12). This finding suggests that ecologic requirements for maintenance could be similar for both viruses.
Land-use changes can affect disease dynamics by modifying the abundance, distribution, behavior, movement, immune response, and community composition of vectors and hosts as well as interactions between vectors and hosts (13). In Argentina, the expansion of agriculture into native ecosystems has generated great modifications of the landscape and the biological communities that inhabit these regions. Specifically, because of the aptitude of its soils, the Pampean region, located in the central-eastern part of Argentina, is one of the areas most greatly modified by human activities. This area has almost entirely been converted to large-scale agricultural land, which in turn has generated changes in the abundance of small mammals and birds (14). However, some species of rodents and native doves have successfully adapted to these changes and, because of their abundance, are considered agricultural pests (15). The large populations of several columbid species, such as eared doves, Picui ground doves, and spot-winged pigeons (Patagioenas maculosa), could generate appropriate ecologic conditions for increased SLEV and WNV activity. In this context, our goal was to study the exposure of free-ranging bird communities to SLEV and WNV and to evaluate environmental and biological factors potentially associated with that exposure in agroecosystems in the Pampean region of Argentina.
Bird capture, manipulation, banding, and blood sampling were authorized by the Direction of Natural Resources belonging to the Subsecretary of Agrarian Affairs from the Ministry of Production of La Pampa Province. Birds were handled according to the guidelines for the use of wild birds in research elaborated by the Ornithological Council (https://birdnet.org/wp content/uploads/2017/07/guc3adas-para-la-utilizacion-de-aves-silvestres-en-investigacic3b3n.pdf). Field studies did not involve endangered or protected species.
Study Area and Sampling Sites
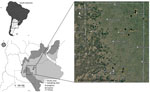
We conducted this study in the northeastern region of La Pampa Province, Argentina, during the period of arbovirus activity (February–April) in 2017–2019. Within the study area, we selected sampling sites for bird captures randomly and included only those with permission from land owners and a minimum distance of 2,000 meters between each other, leading to a total of 12 sampling sites (Figure 1). The study area was formerly part of the Pampean grasslands ecoregion but has been entirely transformed to agriculture. The Pampean grasslands was a vast treeless plain covered by a variety of grasses, such as Sorghastrum pellitum and Elionurus muticus (16). In La Pampa Province, this area is now almost completely transformed, dominated by an agricultural exploitation system based on intensive soybean cultivation via direct sowing methods. Wheat (generally alternated with soybean in the same year), sunflowers, and corn are also cultivated, although to a lesser extent; some plots are seminatural or implanted pastures for cattle (17). Toward the center of the province, soybean cultivation is less common and seminatural pastures dominate the landscape, alternating with different crops such as wheat, corn, and sunflowers. This central area also contains some small isolated patches of Caldén (Prosopis caldenia) forest in the transition to the Espinal ecoregion (Figure 1). Across the study area, but more markedly in the northeastern region, settlements are surrounded by non-native tree woodlots (sometimes up to 20–30 hectares), which constitute a key element in the presence and abundance of pest birds, such as eared doves (15). The climate is dry subhumid; rainfall is distributed throughout the year, but the highest monthly precipitation is in the summer (October–March), increasing in a southwest-to-northeast gradient (18).
Bird Collection and Serum Samples
At each site, we operated 7 mist nets for 3 or 4 days during dawn and late afternoon. We banded collected birds with numbered aluminum leg bands displaying the shipping address of the Argentine Museum of Natural Sciences provided by the Aves Argentinas association (https://www.avesargentinas.org.ar). By using a specialized field guide for bird species from Argentina and Uruguay (19), we recorded species, age, sex, and regular morphometric measurements for each bird. We collected blood by jugular (most species) or brachial (columbids) venipuncture, with 27-gauge sterile needles, into plastic tubes containing 0.45 mL or 0.9 mL (according to a sample volume of 0.1 mL or 0.2 mL) of minimum essential medium for a serum dilution of ≈1:10. We held tubes at room temperature for 20–30 min for coagulation and then placed them into coolers. At the laboratory, we centrifuged samples at 5,000 × g for 15 min for serum separation and then stored them at −20°C. Before releasing the birds, we hydrated those sampled with sugar water. We did not collect blood from birds weighing <10 g.
Serologic Assays and Data Interpretation
We analyzed serum samples to detect neutralizing antibodies by using the plaque-reduction neutralization test. We used low-passage strains of SLEV CbaAr-4005 and WNV E/7229/06. The SLEV CbaAr-4005 strain was isolated from Cx. quinquefasciatus mosquitoes collected in Córdoba Province (20), and the WNV E/7229/06 strain was isolated from a dead horse in Buenos Aires Province, Argentina (9).
We considered all serum samples that neutralized >80% of the inoculated plaque-forming units to be positive and subjected samples that were positive for both viruses to titration (21). We prepared 7 serial 2-fold dilutions of serum, resulting in final dilutions of 1:20, 1:40, 1:80, 1:160, 1:320, 1:640, and 1:1,280. We assigned endpoint titers as the reciprocal of the greatest dilution in which >80% of the challenge virus was neutralized. According to experiments that evaluated cross-reaction between SLEV and WNV in heterologous inoculation scenarios in common quail (Coturnix coturnix), which indicated no cross-reaction between SLEV and WNV (A. Diaz, unpub. data), as well as evidence provided by Patiris et al. (22) and Ledermann et al. (23), we considered all serum samples with antibody titers >20 to be positive. Therefore, we considered samples with titers >20 for both viruses to indicate multiple heterologous infections.
Environmental and Biological Data
To determine the influence of different environmental and biological variables on SLEV and WNV seroprevalence, we built a buffer area with a radius of 1.5 km around the sampling sites, within which we calculated the area occupied by various classes of land cover and other variables of interest. We based our buffer of 1.5 km on the dispersal patterns of several mosquitoes of the genus Culex, particularly Cx. quinquefasciatus (24), and some species of territorial birds, such as house sparrows (Passer domesticus), rufous-collared sparrows (Zonotrichia capensis), and rufous horneros (Furnarius rufus). We used SPOT 6 images granted by the National Commission for Space Activities (https://www.argentina.gob/ar/ciencia/conae). On these images we created a shape or layer file on which polygons corresponding to the different classes of land cover were digitized. Within the buffer area, we estimated the area and distances to variables relevant for arbovirus transmission (Table 1). We used the free open software QGIS version 3.4.10 (https://www.qgis.org) for all GIS procedures and analyzed the following environmental variables: agricultural area (which included crops and pasture lands) expressed in square kilometers; tree coverage (which included native forest patches and non-native tree woodlots) expressed in square kilometers; distance to the nearest water body (expressed in kilometers); and distance to the closest urban settlement (expressed in kilometers) (Tables 1, 2). On the basis of previous host competence studies (7), we considered as biological variables the abundance of doves (eared doves, Picui ground doves, and spot-winged pigeons), cowbirds (grayish baywings [Agelaioides badius] and shiny cowbirds [Molothrus bonariensis]), and house sparrows, considering the abundance as the total number of individuals of each species counted on each sampling site. Dove, cowbird, and sparrow abundance was estimated according to observational and acoustic bird counts on each site, for which we used the fixed width transect method of 50 × 200 m and performed 6 transects in each site according to a rarefaction analysis. The 6 transects were randomly distributed to cover as much of the site as possible and were >200 m apart to minimize possible biases by double counting of birds (25). All linear transects were surveyed once by the same single observer. Bird surveys took place during March and April 2018 and 2019, from 6:00 to 10:00 a.m.
Statistical Analyses
We estimated SLEV and WNV activities by means of neutralizing antibody prevalence. We calculated seroprevalence and 95% CIs by using the package binom (26) and the Pearson-Klopper method within R software (https://www.r-project.org). We analyzed associations between sampling sites, bird species, and exposure to SLEV/WNV through generalized linear mixed models (GLMM) with binomial distribution, in which the sampling year was considered as a random factor. We compared seroprevalence values for each virus evaluated in seropositive birds of each species by using the Pearson χ2 test. We considered p values to be significant at a threshold of α = 0.05. We investigated the association between environmental and biological variables and the SLEV/WNV seroprevalence at each sampling site by using GLMM with binomial error distribution and logit link function, considering the sampling year as a random variable in all models. We evaluated collinearity between explanatory variables by using Pearson correlation with r >0.60 as a limit (Table 1). Because the environmental variables “agricultural area” and “tree coverage” were strongly correlated (r = −0.99), we removed the second variable from the set of models proposed, and because we found the same correlation for the variables “dove abundance” and “cowbird abundance” (r = 0.85), we eliminated “cowbird abundance” from the analyses. The model was selected by using the Akaike information criterion (AIC) and its corrected calculation for small sample sizes (AICc) (27). We compared models by using ΔAICc, which is the difference between the lowest AICc value (as the best of suitable models) and the AICc from all other models. Competing models were those differing by ΔAICc ≤2 from the top model, and Akaike weights (w) were an indication of support for each model. We evaluated the support for the performance of individual predictor variables by summing the AICc weight of a model (wi) across all models that contained the parameter being considered (27). To evaluate the support for parameter estimates, we calculated 95% CIs by using unconditional variances and assumed the considered variable assumed to be significantly associated with the SLEV/WNV seroprevalence when the 95% CI excluded zero (27).
Of the 1,019 free-ranging birds belonging to 44 species collected and sampled, seroprevalence rates were 5.8% (60/1,019) for SLEV and 2.1% (21/1,019) for WNV. Neutralizing antibody titers were >20 for both viruses for 12 birds, which were thus considered to have multiple heterologous infections. Of the 12 sites sampled, birds were seropositive for SLEV at 9 sites, for WNV at 7, and for both viruses at 6 (Table 2, Figure 2).
The GLMM performed to analyze the associations between the sampling sites, avian species, and exposure to SLEV/WNV, showed that sampling site was a significant variable affecting seroprevalence of SLEV (p = 5.87 × 10–16 and WNV (p = 0.0012); seroprevalence for both viruses was highest at sites in the northern area (Figures 1, 2). However, bird species did not significantly influence seroprevalence of SLEV (p = 0.50) or WNV (p = 0.72).
Birds of 17 species were seropositive for SLEV and of 8 species for WNV. Species most exposed to SLEV were house wrens (Troglodytes aedon), chalk-browed mockingbirds (Mimus saturninus), monk parakeets (Myiopsitta monachus), eared doves, and house sparrows (Table 3), whereas those most exposed to WNV were monk parakeets, rufous horneros, and grayish baywings. We found no significant statistical difference between the viruses among seropositive birds of different species, except for house sparrows (p = 0.0004).
The best model explaining the variation in SLEV seroprevalence included the agricultural area as an explanatory variable (wi = 0.44; Table 4). SLEV seroprevalence increased with agricultural area (Table 5). Odds ratio for this model was 1.97, which means that for each unit of increase in agricultural area size, SLEV seroprevalence increased an average of 1.97 times. The model that best explained the variation in WNV seroprevalence included the distance to the nearest water body and agricultural area as explanatory variables (wi = 0.37; Table 6), but neither of the 2 variables was statistically significant to explain the variation in WNV seroprevalence because both 95% CIs included zero (Table 7).
Our estimations of 6% SLEV and 2% WNV seroprevalence in avian hosts in agroecosystems of La Pampa Province are similar to those detected in and around Córdoba city, Argentina (SLEV 7.73%; WNV 1.47%) (21). Composition of biological communities in Córdoba are similar to those in this study.
The species of birds that were infected in the agroecosystems differed according to viruses studied and differed from those found infected by other research conducted in Argentina (21,28). In our study, the species of birds most infected with SLEV belonged to the families Troglodytidae (house wrens), Mimidae (chalk-browed mockingbirds), and Passeridae (house sparrows), although in other studies of similar characteristics and conducted in temperate and subtropical regions of Argentina, the species most infected with this virus belonged to the families Columbidae, Furnariidae, Icteridae, and Tyrannidae (21,28). For WNV, the most infected birds in our study were rufous horneros, which had already been highlighted as maintenance hosts for WNV in central Argentina (21), and monk parakeets, for which WNV infection had not been detected in other studies. One of the main amplifying hosts for SLEV in the United States and for WNV in Europe is the house sparrow (29,30). In previous studies conducted in the northeastern region of Argentina, SLEV seropositivity was not detected in >200 serum samples collected from house sparrows (28). Moreover, in urbanized temperate areas of the central region of Argentina, such as Córdoba, seroprevalence rates for house sparrows have been low for both viruses (3.92% for SLEV, 1.96% for WNV) (21). Furthermore, although the host competence index value for house sparrows is low (7), their high abundance and high exposure to SLEV observed in our study would indicate an efficient role as amplifying hosts for SLEV in agricultural areas of La Pampa Province. A possible explanation for the differences observed among the exposed bird species of and between disturbed environments (agricultural and urban) could be the presence of different vector mosquito species for the viruses evaluated with different host-feeding preferences. Changes in land use could also modify the host-seeking behavior of mosquitoes affecting avian host exposure to vectored viruses (31).
Although seroprevalence values in our study were low, seropositive bird species are resident and seropositive birds were detected during the 3 years sampled. This finding probably indicates endemic circulation for both viruses in this region of Argentina. Previous study of SLEV and WNV activities recorded in Pampean agricultural systems also showed low levels of exposure but in a particular group of birds, the birds of prey (32).
In our study, SLEV seroprevalence was positively associated with the agricultural area, and thus, inversely correlated by tree cover. In other studies, contrary to our results, SLEV infection in humans has been positively associated with proximity to areas with highly productive vegetation cover estimated by the Normalized Difference Vegetation Index (33,34), low density urban construction, and the distance to agricultural fields (34). These differences could be explained because the variables of interest differ (SLEV infections in humans vs. seroprevalence among birds) and the explanatory variables were also considered differently. In our study, we considered the area occupied by agricultural activities and tree coverage within a buffer of interest; in the other studies, researchers considered the distances between cases of SLEV infection in humans and the environmental variables. In turn, the differences found could also result from the fact that the tree coverage in our study area is mostly characterized by planted nonnative tree woodlots, which are inherently different from the green spaces or patches of native forest that characterized the vegetation in other studies.
The model that best explained the variation in WNV seroprevalence included the distance to the nearest water body and agricultural area as explanatory variables, but neither of the 2 variables explained the variation in WNV seroprevalence with statistical significance. Land use effect on WNV activity has been extensively studied, at least in the United States (35–41). Studies have shown that the abundance and distribution patterns of the mosquito vector are key factors in determining virus activity; and these, in turn, are greatly affected by land use. For example, in the northeastern United States, where the main vectors are Cx. pipiens and Cx. quinquefasciatus mosquitoes, urbanization positively affects the incidence of WNV disease in humans (35), whereas on the west coast of the United States, where the most efficient vectors are Cx. tarsalis mosquitoes, the main land cover types associated with increased WNV activity are agricultural irrigated areas, such as rice fields and orchards (40).
Anthropogenic activities are among the most influential factors affecting emergence of infectious diseases, particularly viral vectorborne zoonoses. Viruses carried by Aedes mosquitoes (e.g., chikungunya, dengue, and Zika viruses) are positively affected by urbanization as the main breeding substrates of Ae. aegypti and Ae. albopictus mosquito vectors, which become highly abundant in those anthropogenic and urban habits (42,43). However, for viruses carried by Culex mosquitoes (e.g., Japanese encephalitis virus, WNV, SLEV, and Usutu virus), how anthropogenic changes affect virus activity is not well known. The generalist host-feeding and host-seeking behavior and wide tolerance for rearing sites of the Culex mosquito vectors make it difficult to determine the effect of land use on the activity of Culex mosquitoborne viruses.
Our findings suggest that modified ecosystems, such as agroecosystems in La Pampa Province, have the environmental and biological factors necessary for maintaining and amplifying re-emerging viruses such as SLEV and WNV. However, our study did not analyze the change in land use but rather focused on how the current elements of the already modified landscape influence biological communities and, consequently, SLEV and WNV activity. The sites considered in this study were limited, and the environmental characterization was conducted extensively without taking into account, for example, the identity of the crops or pastures within the agricultural areas. Furthermore, because the seroprevalence data for birds do not necessarily reflect the place or the time in which they were infected, this information should be used with caution and complemented with studies on viral activity in the mosquito communities that ensures circulation of the virus at a certain time and place. Although further research on the ecology and biology of these viruses is needed to determine how crop production, monoculture areas, and associated landscapes affect vector transmission dynamics of these viruses, we conclude that the Pampean agroecosystems in Argentina affect SLEV seroprevalence among avian hosts, providing evidence of the effect of land use on the activity of arboviruses.
Ms. Mansilla is a PhD student at Colaboratorio de Biodiversidad, Ecología y Conservación), INCITAP-CONICET/ FCEyN-UNLPam, La Pampa, Argentina. Her research interests include ecology of viral zoonoses and biological interactions among vectors, hosts, and viruses.
Acknowledgments
We thank Luis Alberto Mansilla, Lucas Gelid, Giovana Peralta, Diego Gallego, Aitué Farana, Milagros Mansilla, Iara Mansilla, Ana Lia Arias, Mikel Larrea, Amaia Frade, Haizea Otaola, and Ibai Alcelay for their invaluable assistance in the field work as well as the private properties owners who allowed us access to their lands. We thank Brenda Konigheim, Javier Aguilar, and Romina Gallardo for their technical support with cell cultures. We also thank Cindy Kemper who kindly reviewed the manuscript.
This research received funding from Ministerio Nacional de Ciencia y Tecnología de Argentina (PICT 2016-3283, PICT 2018-1172), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-PUE IIBYT 2017), and Secretaría de Ciencia y Tecnología–Universidad Nacional de Córdoba (SECyT-UNC). A.P.M. holds a doctoral scholarship from Consejo Nacional de Investigaciones Científicas y Técnicas.
References
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–9. DOIPubMedGoogle Scholar
- Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. Emerging arboviruses: Why today? One Health. 2017;4:1–13. DOIPubMedGoogle Scholar
- Venkat H, Krow-Lucal E, Hennessey M, Jones J, Adams L, Fischer M, et al. Concurrent outbreaks of St. Louis encephalitis virus and West Nile virus disease—Arizona, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1349–50.PubMedGoogle Scholar
- White GS, Symmes K, Sun P, Fang Y, Garcia S, Steiner C, et al. Reemergence of St. Louis encephalitis virus, California, 2015. Emerg Infect Dis. 2016;22:2185–8. DOIPubMedGoogle Scholar
- Diaz A, Coffey LL, Burkett-Cadena N, Day JF. Reemergence of St. Louis encephalitis virus in the Americas. Emerg Infect Dis. 2018;24:2150–7. DOIPubMedGoogle Scholar
- Beranek MD, Quaglia AI, Peralta GC, Flores FS, Stein M, Diaz LA, et al. Culex interfor and Culex saltanensis (Diptera: Culicidae) are susceptible and competent to transmit St. Louis encephalitis virus (Flavivirus: Flaviviridae) in central Argentina. Trans R Soc Trop Med Hyg. 2020;114:725–9. DOIPubMedGoogle Scholar
- Díaz A, Flores FS, Quaglia AI, Contigiani MS. Evaluation of Argentinean bird species as amplifying hosts for St. Louis encephalitis virus (Flavivirus, Flaviviridae). Am J Trop Med Hyg. 2018;99:216–21. DOIPubMedGoogle Scholar
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–73. DOIPubMedGoogle Scholar
- Morales MA, Barrandeguy M, Fabbri C, Garcia JB, Vissani A, Trono K, et al. West Nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis. 2006;12:1559–61. DOIPubMedGoogle Scholar
- Adrián Diaz L, Komar N, Visintin A, Dantur Juri MJ, Stein M, Lobo Allende R, et al. West Nile virus in birds, Argentina. Emerg Infect Dis. 2008;14:689–91. DOIPubMedGoogle Scholar
- Giayetto O, Beranek MD, Nazar FN, Diaz A. Dose dependence of susceptibility and transmission for an Argentinean West Nile virus strain in local Culex pipiens quinquefasciatus (Diptera: Culicidae). Trans R Soc Trop Med Hyg. 2021;115:1066–9. DOIPubMedGoogle Scholar
- Diaz A, Flores FS, Contigiani MS. Viremia profiles and host competence index for West Nile virus (Flavivirus, Flaviviridae) in three autochthonous birds species from Argentina. J Ornithol. 2011;152:21–5. DOIGoogle Scholar
- Gottdenker NL, Streicker DG, Faust CL, Carroll CR. Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth. 2014;11:619–32. DOIPubMedGoogle Scholar
- Bilenca D, Codesido M, González Fischer C. Change in the Pampean Fauna [in Spanish]. Ciencia Hoy. 2008;18:8–17.
- Codesido M, Zufiaurre E, Bilenca D. Relationship between pest birds and landscape elements in the Pampas of Central Argentina. Emu. 2015;115:80–4. DOIGoogle Scholar
- Oyarzabal M, Clavijo J, Oakley L, Biganzoli F, Tognetti P, Barberis I, et al. Unidades de vegetación de la Argentina. Ecol Austral. 2018;28:40–63. DOIGoogle Scholar
- Stella CA, Pall JL, Bernardos J. True bugs (Hemiptera: Heteroptera) associated with soybean (Glycine max (L.) Merr.) in southern cone. Munis Entomol Zool. 2017;12:380–8.
- Cano E, Casagrande G, Conti H, Salazar Lea Plaza J, Peña Zubiarte C, Maldonado Pinedo D, et al. Integrated inventory of the natural resources of the province of La Pampa. Climate, geomorphology, soil and vegetation [in Spanish]. Buenos Aires, Argentina: National University of La Pampa, National Institute of Agricultural Technology; 1980.
- Narosky T, Yzurieta D. Birds of Argentina & Uruguay: a field guide total edition [in Spanish]. 16th ed. Buenos Aires, Argentina: Vazquez Mazzini Editors; 2010.
- Diaz A, Ré V, Almirón WR, Farías A, Vázquez A, Sanchez-seco MP, et al. Genotype III SLEV outbreak, Argentina, 2005. Emerg Infect Dis. 2006;12:2005–7. DOIGoogle Scholar
- Diaz LA, Quaglia AI, Konigheim BS, Boris AS, Aguilar JJ, Komar N, et al. Activity patterns of St. Louis encephalitis and West Nile viruses in free ranging birds during a human encephalitis outbreak in Argentina. PLoS One. 2016;11:
e0161871 . DOIPubMedGoogle Scholar - Patiris PJ, Oceguera LF III, Peck GW, Chiles RE, Reisen WK, Hanson CV. Serologic diagnosis of West Nile and St. Louis encephalitis virus infections in domestic chickens. Am J Trop Med Hyg. 2008;78:434–41. DOIPubMedGoogle Scholar
- Ledermann JP, Lorono-Pino MA, Ellis C, Saxton-Shaw KD, Blitvich BJ, Beaty BJ, et al. Evaluation of widely used diagnostic tests to detect West Nile virus infections in horses previously infected with St. Louis encephalitis virus or dengue virus type 2. Clin Vaccine Immunol. 2011;18:580–7. DOIPubMedGoogle Scholar
- Medeiros MCI, Boothe EC, Roark EB, Hamer GL. Dispersal of male and female Culex quinquefasciatus and Aedes albopictus mosquitoes using stable isotope enrichment. PLoS Negl Trop Dis. 2017;11:
e0005347 . DOIPubMedGoogle Scholar - Bibby C, Jones M, Marsden S. Expedition field techniques: bird surveys. London: Royal Geographical Society; 1998.
- Dorai-Raj S. binom: Binomial confidence intervals for several parameterizations. R package version 1.1–1 [cited 2021 Nov 3]. https://cran.r-project.org/web/packages/binom/binom.pdf
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. Ecological Modelling. New York: Springer; 2002.
- Monath TP, Sabattini MS, Pauli R, Daffner JF, Mitchell CJ, Bowen GS, et al. Arbovirus investigations in Argentina, 1977-1980. IV. Serologic surveys and sentinel equine program. Am J Trop Med Hyg. 1985;34:966–75. DOIPubMedGoogle Scholar
- Reisen WK. Epidemiology of St. Louis encephalitis virus. Adv Virus Res. 2003;61:139–83. DOIPubMedGoogle Scholar
- Del Amo J, Llorente F, Figuerola J, Soriguer RC, Moreno AM, Cordioli P, et al. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro-Mediterranean and North American origins. Vet Res (Faisalabad). 2014;45:33. DOIPubMedGoogle Scholar
- Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Effect of land-use changes on the abundance, distribution, and host-seeking behavior of Aedes arbovirus vectors in oil palm-dominated landscapes, southeastern Côte d’Ivoire. PLoS One. 2017;12:
e0189082 . DOIPubMedGoogle Scholar - Mansilla AP, Solaro C, Orozco-Valor PM, Grande JM, Sarasola JH, Diaz A. Exposure of raptors in central Argentina to St. Louis encephalitis and West Nile viruses. J Raptor Res. 2020;54:279–86. DOIGoogle Scholar
- Rotela CH, Spinsanti LI, Lamfri MA, Contigiani MS, Almirón WR, Scavuzzo CM. Mapping environmental susceptibility to Saint Louis encephalitis virus, based on a decision tree model of remotely-sensed data. Geospat Health. 2011;6:85–94. DOIPubMedGoogle Scholar
- Vergara Cid C, Estallo EL, Almirón WR, Contigiani MS, Spinsanti LI. Landscape determinants of Saint Louis encephalitis human infections in Córdoba city, Argentina during 2010. Acta Trop. 2013;125:303–8. DOIPubMedGoogle Scholar
- Bowden SE, Magori K, Drake JM. Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am J Trop Med Hyg. 2011;84:234–8. DOIPubMedGoogle Scholar
- Bradley CA, Gibbs SEJ, Altizer S. Urban land use predicts West Nile virus exposure in songbirds. Ecol Appl. 2008;18:1083–92. DOIPubMedGoogle Scholar
- Brown HE, Childs JE, Diuk-Wasser MA, Fish D. Ecological factors associated with West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2008;14:1539–45. DOIPubMedGoogle Scholar
- Chuang TW, Hockett CW, Kightlinger L, Wimberly MC. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724–31. DOIPubMedGoogle Scholar
- Ezenwa VO, Milheim LE, Coffey MF, Godsey MS, King RJ, Guptill SC. Land cover variation and West Nile virus prevalence: patterns, processes, and implications for disease control. Vector Borne Zoonotic Dis. 2007;7:173–80. DOIPubMedGoogle Scholar
- Kovach TJ, Kilpatrick AM. Increased human incidence of West Nile virus disease near rice fields in California but not in southern United States. Am J Trop Med Hyg. 2018;99:222–8. DOIPubMedGoogle Scholar
- Miramontes R Jr, Lafferty WE, Lind BK, Oberle MW. Is agricultural activity linked to the incidence of human West Nile virus? Am J Prev Med. 2006;30:160–3. DOIPubMedGoogle Scholar
- Wilke ABB, Chase C, Vasquez C, Carvajal A, Medina J, Petrie WD, et al. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci Rep. 2019;9:15335. DOIPubMedGoogle Scholar
- Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Urbanization is a main driver for the larval ecology of Aedes mosquitoes in arbovirus-endemic settings in south-eastern Côte d’Ivoire. PLoS Negl Trop Dis. 2017;11:
e0005751 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 06, 2022
Table of Contents – Volume 28, Number 7—July 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Adrián Diaz, Instituto de Virología “Dr. J. M. Vanella,” Laboratorio de Arbovirus, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, Enfermera Gordillo Gómez s/n, CPX5016GCA, Córdoba, Argentina
Top