Volume 28, Number 7—July 2022
Research
Self-Reported and Physiologic Reactions to Third BNT162b2 mRNA COVID-19 (Booster) Vaccine Dose
Figure 3
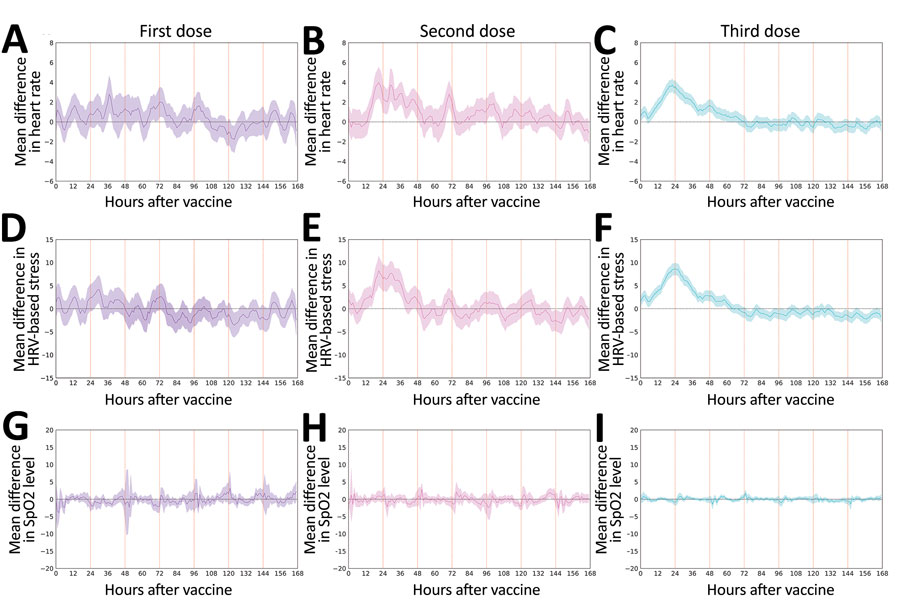
Figure 3. Changes in objective physiologic indicators measured through smartwatch for self-reported and physiologic reactions to BNT162b2 (Pfizer, https://www.pfizer.com) mRNA coronavirus disease vaccine doses. Mean difference are shown for smartwatch-recorded heart rate (A‒C), HRV-based stress (D‒F), and SpO2 (G‒I) after the first, second, and third dose compared with their baseline levels. Mean values are indicated by solid lines; 90% CIs are indicated as shaded regions. Horizontal dashed line indicates no change compared with baseline levels, and vertical lines indicate 24-hour periods. HRV, heart-related variability; SpO2, blood oxygen saturation level.
1These authors contributed equally to this article.
Page created: April 12, 2022
Page updated: June 18, 2022
Page reviewed: June 18, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.