Volume 28, Number 7—July 2022
Research Letter
Bagaza Virus in Wild Birds, Portugal, 2021
Abstract
Bagaza virus emerged in Spain in 2010 and was not reported in other countries in Europe until 2021, when the virus was detected by molecular methods in a corn bunting and several red-legged partridges in Portugal. Sequencing revealed high similarity between the 2021 strains from Portugal and the 2010 strains from Spain.
Bagaza virus (BAGV) is a single-stranded, positive-sense RNA virus. The virus belongs to the mosquitoborne cluster of the genus Flavivirus, family Flaviviridae, which includes such other emerging pathogens as West Nile, Japanese encephalitis, dengue, Zika, and yellow fever viruses, all of which are associated with neurologic disease in animals and humans and have zoonotic potential (1). BAGV was first isolated in 1966 from a pool of Culex species mosquitoes in the Bagaza District of Central African Republic and was detected subsequently in several species of mosquitoes. The first BAGV-associated deaths in vertebrates were detected in Spain, in 2010, in red-legged partridges (Alectoris rufa) and ring-necked pheasants (Phasianus colchicus) (2) and then, in 2016, in Himalayan monal pheasants (Lophophorus impejanus) in South Africa (3).
BAGV infection causes neurologic disease in red-legged partridges, gray partridges (Perdix perdix), ring-necked pheasants, and, to a lesser degree, in common wood pigeons (Columba palumbus) (1–6). Estimated mortality rates range from 23% to 30% in naturally and experimentally infected red-legged partridges (5,7); rates are higher (up to 40%) in experimentally infected gray partridges (6) and lower rates in pheasants and columbiformes (4,7). We describe a BAGV outbreak in Portugal in autumn 2021, associated with abnormal fatalities in red-legged partridges and 1 corn bunting (Emberiza calandra).
On September 1, 2021, three red-legged partridges were found dead in Serpa, southern Portugal. From September through mid-October, 9 partridges and 1 corn bunting were found dead in the same area (Appendix Table). Local reports emerged of partridges displaying neurologic signs compatible with potential viral infection, such as disorientation and motor incoordination. Twelve of the 13 birds were necropsied. Laboratory examinations and preliminary diagnoses were conducted at the Research Institute in Hunting Resources (Ciudad Real, Spain) and at the Center for Research on Biodiversity and Genetic Resources (InBIO Laboratório Associado, Vairão, Portugal). Official diagnosis was determined at the National Institute of Agrarian and Veterinary Research, I.P. (Lisbon, Portugal). Growing feathers were collected from 30 partridges live-trapped in the same area on October 3.
Researchers conducted molecular detection by using RNA extracted from various sampling points (feather pulp, brain, heart, kidney, spleen, and intestine) and followed 2 strategies targeting different regions of the BAGV genome (nonstructural 2b, nonstructural 5 [NS5], and 3′ nontranslated region) (Appendix Table); first, a duplex quantitative reverse transcription PCR (RT-PCR) for the simultaneous and differential detection of Japanese encephalitis and Ntaya flavivirus serocomplexes (8), and second, a uniplex quantitative RT-PCR specific for the NS5 coding region of BAGV (9). The researchers used conventional nested RT-PCR for sequencing to target part of the NS5 gene (10) and an in-house RT-PCR (developed at the National Institute of Agrarian and Veterinary Research) to target part of the NS2b gene (Appendix Table).
Out of the 12 necropsied birds, 8 red-legged partridges and 1 corn bunting (75%) tested positive for BAGV, as did 4 of 30 live-captured red-legged partridges (13.3%) (Appendix Table). The 108 bp sequences obtained from duplex quantitative RT-PCR from partridge 9 and the corn bunting showed 100% similarity with the 3′ nontranslated region of the BAGV reference strain (GenBank accession no. HQ644143) detected in the 2010 outbreak in Spain (Appendix Table). In comparing the NS5 regions, researchers found very high similarities with HQ644143 in the 110 base pair sequences obtained from 6 partridges by nested RT-PCR (99.1%) and in the 171 base pair sequences taken from 2 partridges by RT-PCR (98.8%).
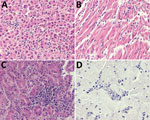
Upon necropsy, all birds were in good body condition, suggesting an acute disease course. Histopathology, albeit hampered by autolysis and freezing artifacts, revealed lymphoid depletion in the spleen and severe congestion, moderate to abundant diffuse mononuclear inflammatory infiltrates, and focal necrosis in all tissues. The heart, brain, kidney, and liver were the most affected organs (Figure).
This work confirms BAGV emergence in Portugal, in autumn 2021, associated with abnormal fatalities in red-legged partridges. Active circulation of BAGV was also evidenced in the studied region, where 13.3% of live-captured red-legged partridges testing positive for BAGV, even though ecologic and demographic studies are required to determine the extent and magnitude of the outbreak. Substantial population decline in the red-legged partridge can be anticipated in this region of Portugal on the basis of the mortality rate previously estimated for this species (4,7). The fatal case in a songbird, the corn bunting, suggests that BAGV might have a broader spectrum and effect in wild bird species. This finding, combined with the small size of the analyzed sequences, suggests the need for further research to identify the vectors for BAGV in Portugal and their role in the epidemiology of the disease, and elucidate the phylogenetic relationships between the 2021 strains in Portugal and 2010 strains in Spain against known BAGV strains.
No conclusions can be made from this research regarding the origin of this infection. However, the introduction of the virus in Portugal might be linked to persistence of the disease and migration of infected wild birds from North Africa or Spain.
Mr. Queirós is a junior researcher at CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, InBIO Laboratório Associado, BIOPOLIS Program in Genomics, Biodiversity and Land Planning, University of Porto. His research interests include the epidemiology of emerging infectious diseases in wildlife species and their interface with livestock and humans, as well as population genetics and phylogeography.
Acknowledgment
We thank the personnel from the hunting sector for their help during field work (sampling and capturing) and Fernanda Simões for the sex determination of partridges 7 and 8 by molecular methods.
References
- Benzarti E, Linden A, Desmecht D, Garigliany M. Mosquito-borne epornitic flaviviruses: an update and review. J Gen Virol. 2019;100:119–32. DOIPubMedGoogle Scholar
- Agüero M, Fernández-Pinero J, Buitrago D, Sánchez A, Elizalde M, San Miguel E, et al. Bagaza virus in partridges and pheasants, Spain, 2010. Emerg Infect Dis. 2011;17:1498–501. DOIPubMedGoogle Scholar
- Steyn J, Botha EM, Lourens C, Coetzer JAW, Venter M. Bagaza virus in Himalayan monal pheasants, South Africa, 2016–2017. Emerg Infect Dis. 2019;25:2299–302. DOIPubMedGoogle Scholar
- Gamino V, Gutiérrez-Guzmán AV, Fernández-de-Mera IG, Ortíz JA, Durán-Martín M, de la Fuente J, et al. Natural Bagaza virus infection in game birds in southern Spain. Vet Res (Faisalabad). 2012;43:65. DOIPubMedGoogle Scholar
- Llorente F, Pérez-Ramírez E, Fernández-Pinero J, Elizalde M, Figuerola J, Soriguer RC, et al. Bagaza virus is pathogenic and transmitted by direct contact in experimentally infected partridges, but is not infectious in house sparrows and adult mice. Vet Res (Faisalabad). 2015;46:93. DOIPubMedGoogle Scholar
- Cano-Gómez C, Llorente F, Pérez-Ramírez E, Soriguer RC, Sarasa M, Jiménez-Clavero MÁ. Experimental infection of grey partridges with Bagaza virus: pathogenicity evaluation and potential role as a competent host. Vet Res (Faisalabad). 2018;49:44. DOIPubMedGoogle Scholar
- García-Bocanegra I, Zorrilla I, Rodríguez E, Rayas E, Camacho L, Redondo I, et al. Monitoring of the Bagaza virus epidemic in wild bird species in Spain, 2010. Transbound Emerg Dis. 2013;60:120–6. DOIPubMedGoogle Scholar
- Elizalde M, Cano-Gómez C, Llorente F, Pérez-Ramírez E, Casades-Martí L, Aguilera-Sepúlveda P, et al. A duplex quantitative real-time reverse transcription-PCR for simultaneous detection and differentiation of flaviviruses of the Japanese encephalitis and Ntaya serocomplexes in birds. Front Vet Sci. 2020;7:203. DOIPubMedGoogle Scholar
- Buitrago D, Rocha A, Tena-Tomás C, Vigo M, Agüero M, Jiménez-Clavero MA. Real-time fluorogenic reverse transcription polymerase chain reaction assay for the specific detection of Bagaza virus. J Vet Diagn Invest. 2012;24:959–63. DOIPubMedGoogle Scholar
- Sánchez-Seco MP, Rosario D, Domingo C, Hernández L, Valdés K, Guzmán MG, et al. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J Virol Methods. 2005;126:101–9. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: June 09, 2022
Table of Contents – Volume 28, Number 7—July 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
João Queirós, CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, InBIO Laboratório Associado, BIOPOLIS Program in Genomics, Biodiversity and Land Planning, Campus de Vairão, Universidade do Porto, 4485-661 Vairão, Portugal
Top