Volume 29, Number 1—January 2023
Research Letter
Short-Finned Pilot Whale Strandings Associated with Pilot Whale Morbillivirus, Brazil
Cite This Article
Citation for Media
Abstract
Cetacean morbillivirus (CeMV) causes illness and death in cetaceans worldwide; the CeMV strains circulating in the Southern Hemisphere are poorly known. We detected a pilot whale CeMV strain in 3 short-finned pilot whales (Globicephala macrorhynchus) stranded in Brazil during July–October 2020. Our results confirm this virus circulates in this species.
Cetacean morbillivirus (CeMV; family Paramyxoviridae, genus Morbillivirus) is an important cause of illness and death in cetaceans (1). The genus Morbillivirus comprises 2 lineages: CeMV-1, which includes dolphin morbillivirus (DMV), porpoise morbillivirus (PMV), pilot whale morbillivirus (PWMV), and beaked whale morbillivirus (BWMV) strains; and CeMV-2, comprising the strain detected in Indo-Pacific bottlenose dolphins (Tursiops aduncus) in western Australia, the Fraser’s dolphin morbillivirus (FDMV), and Guiana dolphin morbillivirus (GDMV) strains (1,2). GDMV has been the only strain reported in cetaceans in Brazil (3). Four cases of PWMV have been recorded in pilot whales of the Northern Hemisphere, on the Atlantic coast of the United States and in the Canary Islands, Spain (4,5).
During July–October 2020, four short-finned pilot whales (Globicephala macrorhynchus) stranded in Brazil: 2 in Ceará state (cases 1 and 2) and 2 in Santa Catarina state (cases 3 and 4). All the animals stranded alive and died within 24 hours (Appendix Figure 1). We performed standard necropsies and collected tissue samples, which we fixed in 10% buffered formalin for histopathology or froze at −20°C or −80°C for molecular analysis.
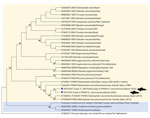
We performed RNA extractions of all available tissues with TRIzol-LS (Life Technologies Corporation, https://www.thermofisher.com). We performed a morbillivirus 2-step reverse transcription nested PCR to amplify the phosphoprotein gene (6). After DNA extraction with the QIAGEN Blood & Tissue Kit (QIAGEN, https://www.qiagen.com), we performed herpesvirus detection in lung (n = 2) and liver (n = 4) samples by nested pan-PCRs to amplify DNA polymerase and glycoprotein B genes (7); when those were positive, we tested the remaining available tissues using the same protocols. We calculated percentage of identity among the obtained sequences and the closest ones from GenBank/EMBL/DDBJ based on p-distance. We used MEGA7 (https://www.megasoftware.net) to construct the phylogram (Figure).
Three animals, cases 1, 3 and 4, were morbillivirus-positive, amplified in central nervous system, lung, and pulmonary lymph node samples (Table); sequences were submitted to GenBank (case 1, accession no. OP375347; case 3, OP375348; case 4, OP375349). Sequences from case 1 and 3 were identical and had a single nucleotide missense mutation (99.7% nt identity, 99.2% aa identity) when compared to the sequence from case 4. The sequences from cases 1 and 3 presented the highest nucleotide (99%) and amino acid identities (96.9%) with a PWMV sequence identified in 2 pilot whales in the Canary Islands, Spain (GenBank accession nos. KT006289 [animal 1], KT006290, and KT006291 [animal 2]). The sequence from case 4 had the highest nucleotide (99.2%) and amino acid similarities (97.7%) to the same PWMV sequences. Our sequences clustered with other PWMV sequences (Figure). In addition, we detected an alphaherpesvirus by the DNA polymerase protocol in a lung sample from case 3 (GenBank accession no. OP341880). The remaining tissue samples of case 3 (cerebellum, kidney, mesenteric lymph node, spleen, and liver) were herpesvirus-negative by PCR. The obtained herpesvirus has the highest similarity (99.5% nt identity, 100% aa identity) to an alphaherpesvirus obtained in a striped dolphin (Stenella coeruleoalba) from Spain (GenBank accession no. GQ888671).
The general health of the CeMV-positive animals was poor, and all were undernourished. We compared the main pathologic findings in these animals to all other cases of PWMV strain reported in the literature (Table).
Pilot whales are susceptible to DMV and PWMV; DMV caused atypical pilot whale deaths in the Mediterranean Sea (6). By contrast, 4 cases of PWMV infections have been recorded; 1 in New Jersey, USA, and 3 in the Canary Islands, Spain (4–6,9,10). All of them had multiorgan infections (4,5). Case 1 likely had a subacute or systemic CeMV infection characterized by meningomyelitis with gliosis and lymphocytic bronchointerstitial pneumonia. Further studies are necessary to elucidate if cases 3 and 4 manifested an infection similar to the brain-only DMV form or a systemic infection with heterogenic dissemination. The poor nutritional condition observed in all PWMV-positive animals could be the result of decreased foraging capacity caused by encephalitis (1). Case 3 had alphaherpesvirus and CeMV co-infection, a comorbidity previously reported in cetaceans, including pilot whales (5,10); in this case, however, there were no associated herpesviral lesions. All PWMV-positive cetaceans we described were juveniles, which could be associated with maternal passive immunity loss.
The occurrence of pilot whale strandings in 2020 on the coast of Brazil could be considered atypical. Of interest, although case 1 was stranded >3,300 km away from case 3 along the coastline, it had the same PWMV sequence type, which suggests circulation of that type along the coast of Brazil. Further studies are necessary to understand the effects and epidemiology of morbillivirus in cetaceans in the South Atlantic Ocean. However, the high similarity between our sequences and the PWMV detected in the Northern Hemisphere confirms that this strain also circulates in South America pilot whales and might be enzootic in Globicephala sp. whales in the Atlantic Ocean.
Dr. Costa-Silva, a veterinarian specialist in marine mammals, completed this project as part of her PhD project in the Department of Preventive Veterinary Medicine and Animal Health, School of Veterinary Medicine and Animal Science, University of São Paulo.
Acknowledgments
We thank Aquasis, Universidade do Estado de Santa Catarina (UDESC), and Universidade da Região de Joinville (UNIVILLE) for logistic and technical support. We thank the Santos Basin Beach Monitoring Project (Projeto de Monitoramento de Praias da Bacia de Santos, PMP-BS) and Potiguar Basin Beach Monitoring Project (Projeto de Monitoramento de Praias da Bacia Potiguar, PMP-BP), conducted by Petrobrás, licensed by the Brazilian Institute of the Environment and Renewable Natural Resources of the Brazilian Ministry of Environment under ABIO no. 640/2015.
The Coordination for the Improvement of Higher Education Personnel (CAPES), National Council for Technological and Scientific Development (CNPq), and São Paulo Research Foundation (FAPESP) provided financial support. S.C.S. and A.C.E. received PhD fellowships by FAPESP (process nos. 2020/12434-9 and 2016/20956-0). L.B.K. received financial support from FAPESP (no. 2020/12434-9). J.L.C.-D., L.B.K. and M.J.C. are recipients of research productivity fellowships from CNPq (nos. 304999-18, 315619/2021-0, and 313577/2020-0, respectively), C.S. is a recipient of a Juan de la Cierva incorporación fellowship (no. IJC2020-046019-I) and received a postdoctoral grant by FAPESP (no. 2018/25069-7).
References
- Van Bressem MF, Duignan PJ, Banyard A, Barbieri M, Colegrove KM, De Guise S, et al. Cetacean morbillivirus: current knowledge and future directions. Viruses. 2014;6:5145–81. DOIPubMedGoogle Scholar
- West KL, Silva-Krott I, Landrau-Giovannetti N, Rotstein D, Saliki J, Raverty S, et al. Novel cetacean morbillivirus in a rare Fraser’s dolphin (Lagenodelphis hosei) stranding from Maui, Hawai’i. Sci Rep. 2021;11:15986. DOIPubMedGoogle Scholar
- Groch KR, Groch KR, Kolesnikovas CKM, de Castilho PV, Moreira LMP, Barros CRMB, et al. Cetacean morbillivirus in southern right whales, Brazil. Transbound Emerg Dis. 2019;66:606–10. DOIPubMedGoogle Scholar
- Bellière EN, Esperón F, Fernández A, Arbelo M, Muñoz MJ, Sánchez-Vizcaíno JM. Phylogenetic analysis of a new Cetacean morbillivirus from a short-finned pilot whale stranded in the Canary Islands. Res Vet Sci. 2011;90:324–8. DOIPubMedGoogle Scholar
- Sierra E, Fernández A, Suárez-Santana C, Xuriach A, Zucca D, Bernaldo de Quirós Y, et al. Morbillivirus and pilot whale deaths, Canary Islands, Spain, 2015. Emerg Infect Dis. 2016;22:740–2. DOIPubMedGoogle Scholar
- Fernández A, Esperón F, Herraéz P, de Los Monteros AE, Clavel C, Bernabé A, et al. Morbillivirus and pilot whale deaths, Mediterranean Sea. Emerg Infect Dis. 2008;14:792–4. DOIPubMedGoogle Scholar
- Sacristán C, Esperón F, Ewbank AC, Costa-Silva S, Marigo J, Matushima ER, et al. Identification of novel gammaherpesviruses in a South American fur seal (Arctocephalus australis) with ulcerative skin lesions. J Wildl Dis. 2018;54:592–6. DOIPubMedGoogle Scholar
- Taubenberger JK, Tsai MM, Atkin TJ, Fanning TG, Krafft AE, Moeller RB, et al. Molecular genetic evidence of a novel morbillivirus in a long-finned pilot whale (Globicephalus melas). Emerg Infect Dis. 2000;6:42–5. DOIPubMedGoogle Scholar
- Duignan PJ, House C, Geraci JR, Early G, Copland HG, Walsh MT, et al. Morbillivirus infection in two species of pilot whale (Globicephala sp.) from the western Atlantic. Mar Mamm Sci. 1995;11:150–62. DOIGoogle Scholar
- Di Guardo G, Mazzariol S. Cetacean morbillivirus-associated pathology: knowns and unknowns. Front Microbiol. 2016;7:112. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: December 19, 2022
Table of Contents – Volume 29, Number 1—January 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Samira Costa-Silva, Av. Duque de Caxias Norte, 225, Bairro, Jardim Elite (campus da USP), Pirassununga, São Paulo, 13635-900, Brazil
Top