Volume 29, Number 11—November 2023
Dispatch
Erythema Migrans Caused by Borrelia spielmanii, France
Cite This Article
Citation for Media
Abstract
We describe a rare case of early Lyme borreliosis in France caused by Borrelia spielmanii, which manifested as a large erythema chronicum migrans rash. The patient completely recovered after a 15-day course of amoxicillin. Absence of pathognomonic signs prevented distinguishing B. spielmanii from other etiologies as cause in this case-patient.
The causative agents of borreliosis, also known as Lyme disease, in Europe are Borrelia garinii, B. afzelii, and, more rarely, B. burgdorferi sensu stricto. Lyme borreliosis is endemic in France except the southern region. Mean annual incidence of Lyme borreliosis in France may be as high as 84 cases/100,000 persons (1).
B. spielmanii is a rare agent of Lyme borreliosis first isolated in 1993 from a patient with erythema chronicum migrans (ECM) in the Netherlands (2), then well-characterized as a novel genetic variant in 1999 (3). B. spielmanii was described as a new species in 2006 with a type strain isolated from Ixodes ricinus ticks collected from a garden dormouse in the Petite Camargue Alsacienne region in France (4,5). A few human cases were reported from Germany (4), Hungary (6), Czech Republic (7), Denmark, and Slovenia (8); patients in all reported cases exhibited ECM (9). B. spielmanii has been found in a low percentage of ticks feeding on dogs in the United Kingdom, Switzerland, and Belgium and on birds in Poland. Also, ticks in Austria, Denmark, and the Czech Republic (some removed from humans), I. ricinus ticks in the Crimea peninsula (10), as well as in animal tissues from Poland (red fox) (11) and Hungary (hedgehogs), have been shown to carry B. spielmanii (12). No infected humans have been identified France.
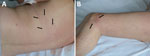
A 60-year-old woman sought treatment on November 15, 2017, for 2 erythematous linear asymptomatic and noninfiltrated bands on her left knee and the left side of her thorax. In August 2017, the woman had noticed an annular erythema initially in the middle and on the left side of her back. The annular erythema had gradually extended to the upper left side of her back to form a single erythematous band (Figure 1, panel A) and progressed to the left knee, also forming an erythematous band (Figure 1, panel B). Both general and skin exams were otherwise unremarkable. We hypothesized that the rash might constitute an unusually large ECM. Because Lyme borreliosis is unknown in that area of southeastern France, we questioned her about her recent travel history. She had spent the week of June 14–20, 2017, in the county of Oise, north of Paris, where Lyme borreliosis is endemic. We performed a punch biopsy on the thoracic erythema band and prescribed oral amoxicillin (1 g 3×/d for 15 d), which resolved the ECM.
We screened a serum sample from the patient using an enzyme-linked immunoassay (Liaison Borrelia burgdorferi; DiaSorin, https://www.diasorin.com), which revealed presence of IgG and absence of IgM for B. burgdorferi sensu lato. Western blotting using LymeCheck Optima IgG & IgM (Biosynex, https://www.biosynex.com) revealed presence of Borrelia spp.–specific IgG for p100, VlsE, p58, and p41 antigens and a faint band for B. spielmanii–specific ospC antigen (Appendix Figure). Two weak bands showed IgM for B. spielmanii p100 and p41 antigens (Appendix Figure).
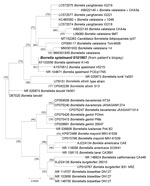
We amplified portions of 16S rRNA (13) and ospA (5) borrelial genes from the biopsy sample. BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi) of sequenced amplicons showed 100% identity of the biopsy sequences with strain A14S of B. spielmanii for both the 910 bp–long portion of 16S rRNA gene (Genbank accession no. AF102056) and 260 bps-long amplicon of ospA (Genbank accession no. CP001469). We deposited our sequences into Genbank (accession nos. OR192893 and OR234396). The phylogenic tree (Figure 2) showed that the sequence obtained from the patient clusters with other B. spielmanii strains.
Previously, only B. azfelii, B. garinii, and B. burgdoferi sensu stricto had been identified from patients in France (14). Our patient manifested a rare clinical form of ECM, with a large erythema migrans across her body, extending from her upper back to her knee. However, the unusually large size might have resulted from delays in seeking treatment and diagnosis, so that particular clinical manifestation might not be specific to B. spielmanii.
We reviewed available literature on clinical descriptions of human cases of B. spielmanii infection and found only 2 published case reports. A 69-year-old woman from Slovenia showed skin manifestations described as redness, mild local itching, burning, and pain on the left knee and later a 24 × 20 cm ring-like lesion on the left thigh (8), but she had no identified tick bite. In a second case, a 42-year-old woman from Hungary exhibited an ECM 10 cm in diameter on her knee (6). Clinical manifestations were missing in other case reports (3,9), in which only human isolates were described. In 1 study, B. spielmanii was detected in isolates from 4/242 patients with ECM from Germany and Slovenia (9). However, in that study, 3 of the 4 patients infected with B. spielmanii lived in Munich where a higher proportion of ticks were positive for that pathogen. This finding argues for sporadic occurrences of the infection in other locations.
In summary, our study has contributed more data on Borrelia spp. as potential causes of Lyme disease, prompting need for broader surveillance. However, additional well-documented reports on ECM as a manifestation of B. spielmanii are needed to provide new information about the epidemiology of this Borrelia in Europe.
Dr. Del Giudice is a physician in the Infectious Diseases and Dermatology Unit at Bonnet Hospital, Fréjus, France. His primary research interest is skin infections.
Acknowledgment
We thank Mrs. Moussa Hajer for editing the figures, proofreading, and submitting this manuscript.
References
- Figoni J, Chirouze C, Hansmann Y, Lemogne C, Hentgen V, Saunier A, et al.; endorsed by scientific societies. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): prevention, epidemiology, diagnosis. Med Mal Infect. 2019;49:318–34. DOIPubMedGoogle Scholar
- van Dam AP, Kuiper H, Vos K, Widjojokusumo A, de Jongh BM, Spanjaard L, et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–17. DOIPubMedGoogle Scholar
- Wang G, van Dam AP, Dankert J. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with lyme borreliosis. J Clin Microbiol. 1999;37:3025–8. DOIPubMedGoogle Scholar
- Richter D, Schlee DB, Allgöwer R, Matuschka FR. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in Central Europe. Appl Environ Microbiol. 2004;70:6414–9. DOIPubMedGoogle Scholar
- Richter D, Postic D, Sertour N, Livey I, Matuschka FR, Baranton G. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int J Syst Evol Microbiol. 2006;56:873–81. DOIPubMedGoogle Scholar
- Földvári G, Farkas R, Lakos A. Borrelia spielmanii erythema migrans, Hungary. Emerg Infect Dis. 2005;11:1794–5. DOIPubMedGoogle Scholar
- Richtrová E, Míchalová P, Lukavská A, Navrátil J, Kybicová K. Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in urban green areas in Prague. Ticks Tick Borne Dis. 2022;13:
102053 . DOIPubMedGoogle Scholar - Maraspin V, Ruzic-Sabljic E, Strle F. Lyme borreliosis and Borrelia spielmanii. Emerg Infect Dis. 2006;12:1177. DOIPubMedGoogle Scholar
- Fingerle V, Schulte-Spechtel UC, Ruzic-Sabljic E, Leonhard S, Hofmann H, Weber K, et al. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int J Med Microbiol. 2008;298:279–90. DOIPubMedGoogle Scholar
- Nefedova VV, Korenberg EI, Andreĭchuk IV, Gorelova NB, Markov AV, Fadeeva IA, et al. [Genetic characterization of pathogenic Borrelia, group A14S, isolated in Ukraine] [in Russian]. Zh Mikrobiol Epidemiol Immunobiol. 2005;4:23–7.PubMedGoogle Scholar
- Wodecka B, Michalik J, Grochowalska R. Red foxes (Vulpes vulpes) are exposed to high diversity of Borrelia burgdorferi sensu lato species infecting fox-derived Ixodes ticks in west-central Poland. Pathogens. 2022;11:696. DOIPubMedGoogle Scholar
- Szekeres S, Docters van Leeuwen A, Tóth E, Majoros G, Sprong H, Földvári G. Road-killed mammals provide insight into tick-borne bacterial pathogen communities within urban habitats. Transbound Emerg Dis. 2019;66:277–86. DOIPubMedGoogle Scholar
- Raoult D, Ndihokubwayo JB, Tissot-Dupont H, Roux V, Faugere B, Abegbinni R, et al. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet. 1998;352:353–8. DOIPubMedGoogle Scholar
- Lenormand C, Jaulhac B, Debarbieux S, Dupin N, Granel-Brocard F, Adamski H, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans [ACA]): A prospective study of 20 culture- and/or polymerase chain reaction (PCR)-documented cases. J Am Acad Dermatol. 2016;74:685–92. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: October 05, 2023
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Pascal del Giudice, Infectiology and Dermatology Unit, Centre Hospitalier Intercommunal de Fréjus-Saint-Raphaël, 240 Av. de Saint-Lambert, 83600 Fréjus, France
Top