Volume 29, Number 11—November 2023
Research
Prevalence of Undiagnosed Monkeypox Virus Infections during Global Mpox Outbreak, United States, June–September 2022
Cite This Article
Citation for Media
Abstract
Since May 2022, mpox has been identified in 108 countries without endemic disease; most cases have been in gay, bisexual, or other men who have sex with men. To determine number of missed cases, we conducted 2 studies during June–September 2022: a prospective serologic survey detecting orthopoxvirus antibodies among men who have sex with men in San Francisco, California, and a retrospective monkeypox virus PCR testing of swab specimens submitted for other infectious disease testing among all patients across the United States. The serosurvey of 225 participants (median age 34 years) detected 18 (8.0%) who were orthopoxvirus IgG positive and 3 (1.3%) who were also orthopoxvirus IgM positive. The retrospective PCR study of 1,196 patients (median age 30 years; 54.8% male) detected 67 (5.6%) specimens positive for monkeypox virus. There are likely few undiagnosed cases of mpox in regions where sexual healthcare is accessible and patient and clinician awareness about mpox is increased.
Since May 2022, monkeypox virus (MPXV) infections have been detected in 104 countries without endemic disease. Most cases have been among gay, bisexual, or other men who have sex with men (MSM). Because lesions commonly occur on the genitals, mpox was most frequently diagnosed in clinics conducting sexually transmitted infection (STI) screening (1). The diagnosis can be challenging because the mpox rash has been confused with STIs (e.g., herpes simplex virus infection and syphilis), hand-foot-and-mouth disease, varicella zoster virus infection, and even arthropod bites (2–4). In addition to cases being undiagnosed because of diminished clinical suspicion, some cases may have been undiagnosed if patients did not seek care (i.e., because the symptoms were mild and self-limiting or because of poor access to a medical provider). As the global outbreak continued, public health authorities continued to increase awareness of mpox. However, clinicians and public health authorities were concerned that if a high number of cases were missed, the outbreak would be difficult to control. To determine the number of undiagnosed MPXV infections in the United States, we conducted 2 studies during June–September 2022: a prospective serologic surveillance study among MSM who sought sexual health services in San Francisco, California, USA, and a retrospective study of molecular testing of specimens tested for other infectious diseases linked to specific codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), among all populations. Each study used specimens collected during the peak of the outbreak. Our studies were reviewed by the Centers for Disease Control and Prevention (CDC) and were conducted consistent with applicable federal law and CDC policy (e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Prospective Serologic Survey
For the primary recruitment sites for this serologic survey, we selected 3 prominent sexual health clinics (clinics A, B, C) in San Francisco that regularly treat MSM and 1 research clinic in San Francisco (clinic D). Those 4 private and publicly funded clinics encompass an estimated 20,000 MSM patients of varying socioeconomic status, insured rates (2%–85% private, 14%–92% public, 0–40% uninsured), and races and ethnicities within the San Francisco Bay area. Patients entering the 3 sexual health clinics during June 28–August 26, 2022, were given an informational flier in English or Spanish containing a QR code that directed interested patients to a survey to self-screen for inclusion. The flier also stated that participation was voluntary and the decision to enroll would not in any way affect their medical care. Study inclusion was limited to patients who self-reported that they did not have symptoms of mpox (e.g., rash, fever, lymphadenopathy), had never received an mpox diagnosis, were 18–50 years of age (the upper age limit was set to exclude childhood smallpox vaccination in the United States), and did not have a history of smallpox or mpox vaccination. Because most cases detected at that point in the outbreak were in MSM, and to ensure sufficient participation among this population at high risk for mpox, we also excluded cisgender women and persons who did not identify as ever having had male-to-male sexual contact. Participants at clinic D were recruited by a query into the electronic medical record system from HIV and HIV pre-exposure prophylaxis registries; a subset of MSM patients 18–50 years of age were sent an invitation to participate. At clinic arrival, those participants were given the same survey to self-screen. All participants who completed the self-screening questionnaire and were eligible for study inclusion, then completed a brief 7-question electronic survey that asked about factors thought to be associated with risk for mpox during the initial stage of the outbreak that could affect public health action (e.g., travel and exposure history within the past 90 days) (Appendix 1) (3). We collected 5 mL of blood from each participant who completed the questionnaire. Peak IgM is detected 2–3 weeks and IgG 3–5 weeks after exposure to an orthopoxvirus (including primary vaccination with ACAM2000 [second-generation live vaccinia virus vaccine] and JYNNEOS [third-generation live, non-replicating, modified vaccinia Ankara Bavarian Nordic vaccine; https://jynneos.com]), and convalescence has been documented at 7–14 weeks after exposure. Orthopoxvirus IgM is reliably detected 4–56 days and IgG >8 days after rash onset (5). Because IgG persists for several years after orthopoxvirus exposure (6), we chose IgG as the initial screening tool to detect any past orthopoxvirus exposure. To detect recent exposure, we tested positive IgG specimens for IgM. We separated serum by centrifugation, aliquoted the samples, and sent them to CDC for ELISA analysis of orthopoxvirus IgG and, if positive, IgM.
Retrospective Molecular Testing
During the multinational outbreak that began in 2022, mpox was diagnosed by nonvariola orthopoxvirus- and MPXV-specific real-time PCR tests of lesion swab samples (7,8). Before an mpox-specific diagnosis code (B.04) was established, clinical diagnoses and testing were documented with ICD-10-CM codes representing broad symptoms of infection, which were used as a surveillance tool for early identification of potentially undiagnosed infections similar to other diseases (9,10). To evaluate the presence of MPXV within specimens received for other testing, CDC partnered with HealthTrackRx, a private laboratory that receives specimens from a variety of clinics across the United States for infectious disease testing. During June 1–September 2, 2022, CDC deidentified and tested lesion swab specimens associated with ICD-10-CM codes for genital lesions, herpes simplex virus infection, inflammation of the genital region, skin rash, and others that may overlap with symptoms of mpox (Appendix 2) for presence of MPXV DNA by using a clade II–specific PCR (8). After June 27, 2022, HealthTrackRx validated its own mpox clade II–specific assay (8) and continued to test specimens for MPXV that fit the ICD-10-CM codes (Appendix 2). No specimens were excluded; only basic demographic and geographic data and pertinent ICD-10-CM codes that may be associated with mpox were available from the initial test request from the submitting clinician. No information about sexual history was included.
Prospective Serologic Survey
During the study period, ≈8,670 patients were seen at clinics A, B, and C, of which 3,832 (44.2%) were MSM, 18–50 years of age, and may have been eligible for participation. An estimated 6,000 persons from clinic D were eligible for study participation, and 2,400 (40%) were sent an invitation to participate. A total of 398 patients started the survey. Of 358 (87.4%) participants who completed the survey, 133 were excluded for not self-identifying as having male-to-male sexual contact (n = 67), reporting previous receipt of smallpox or mpox vaccination (n = 41), being >50 years of age (n = 18), or reporting a past diagnosis of mpox (n = 7). We collected serum samples from the final sample size of 225 participants. Participant median age was 34 (interquartile range [IQR] 29–42) years. Most (52.9%) eligible participants were non-Hispanic White, and most (87.1%) reported sexual orientation as gay (Tables 1, 2). Twenty-six (11.6%) participants reported known contact with someone with mpox. Recent travel (previous 3 months) was reported by 77 (34.2%); among the 67 who reported a location, 38 (56.7%) had traveled in the United States, 17 (25.4%) to Europe, and 13 (19.4%) to other countries within the Americas. A total of 130 (57.8%) participants had attended a large private or public event (e.g., festivals, parades, weddings, clubs, sex parties). Most (203, 91.2%) participants had >1 sexual contact in the previous month, among which 68 (30.2%) had >5 partners. A total of 65 (28.9%) participants had an immunocompromising condition, most commonly HIV (89.2%; n = 58). Of those who reported HIV, 8 (13.8%) reported a CD4 count <200 cells/mm3 and 9 (15.5%) reported a viral load >200 copies/mL. Among the 47 (20.9%) who reported being ill in the previous 3 months, the most common signs/symptoms were cough, rhinorrhea, sore throat, fever, and chills (participants could report >1 sign/symptom).
Of 225 serum samples tested for orthopoxvirus IgG, 18 (8.0%) were also positive and 3 (1.3%) were positive for orthopoxvirus IgM. Those 3 participants were 20–49 years of age. Two patients denied prior smallpox or mpox vaccination; vaccination status for the third patient was unknown. All 3 participants had traveled in the previous 3 months (2 internationally and 1 domestically), 1 reported attending a large event, and 1 reported having had contact with someone with mpox. All 3 participants reported having had 3–20 sex partners within the previous month. Two participants reported signs/symptoms consistent with mpox in the previous 3 months, including rash, diaphoresis, and lymphadenopathy. One participant had well-controlled HIV (CD4 count >200 cells/μL).
Retrospective Molecular Testing
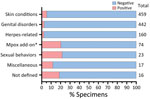
During the study period, MPXV testing was performed for 1,196 patients (median age 30 [IQR 19–46] years); 656 (54.8%) were men. The most common specimen collection sites were arm (24.8%; n = 297), anogenital (18.6%; n = 222), leg (10.1%; n = 121), and unspecified (14.2%; n = 170). The ICD-10-CM codes accompanying specimens were broadly categorized as disorder of the genitals, herpes-related lesions, pruritus, cellulitis, skin conditions, vaginitis, high-risk sexual behavior, mpox, miscellaneous, and not defined. A total of 67 (5.6%) specimens tested positive for MPXV DNA (Figure 1). The dates that the positive specimens had been obtained corresponded to the increase in mpox epidemic curve in the United States (Figure 2). Most MPXV-positive specimens were associated with skin conditions, including ICD-10-CM codes R21 (rash and other nonspecific skin eruption), L98.9 (disorder of skin and subcutaneous tissue, unspecified), L08.89 (other specified local infections of the skin and subcutaneous tissue), and disorders of the genital regions including N48.5 (ulcer of the penis) (Table 3). Among those categories, all specimens with ICD-10-CM codes corresponding to signs/symptoms of pruritis, cellulitis, and vaginitis tested negative for MPXV; no positive specimens were from women. Among the 67 MPXV-positive specimens, 5 (7.3%) ICD-10-CM codes were classified under sexual behavior that places someone at increased STI/HIV risk and 4 (5.8%) under herpes-related lesions. Of the 67 positive specimens, 15 (20.3%) were among 74 specimens that were originally submitted for testing of other infectious organisms but after negative results had been submitted for MPXV testing at provider request.
Most specimens received were from Michigan (12.8%), Georgia (12.0%), Colorado (10.4%), and Florida (9.9%); however, the highest proportion of specimens that tested positive for mpox were from Georgia (24.5%, 35 positive), followed by Missouri (25.0%, 5 positive) and Texas (12.9%, 11 positive) (Table 4). Specimens were also tested on the STI and wound infection PCR panels at HealthTrackRx. Among the specimens testing positive for mpox, only 1 tested positive for other etiologies consistent with contamination (Finegoldia magna, Cutibacterium acnes, and Peptostreptococcus spp).
A total of 21,798 mpox cases were reported in the United States during the peak of the outbreak, June–September 2022, accounting for 72.0% of the total US cases reported as of March 2023. Despite concerns that some cases could be undetected (particularly in the MSM community), potentially preventing outbreak control, the serologic survey identified only 1.3% of MSM patients at high risk for mpox without a known mpox diagnosis who had orthopoxvirus IgM, indicating recent exposure to mpox. That rate of IgM positivity is similar to the 1.4% rate among persons experiencing homelessness in San Francisco during July–October 2022 (11). Mpox was retrospectively detected by PCR in 5.6% of lesion swab samples obtained across the country, suggesting that mpox was probably undiagnosed in a small subset of symptomatic patients during the height of the mpox outbreak in the United States. The highest percentage positivity was among those who reported sexual behavior that places someone at increased for STI/HIV. However, the second highest percentage positivity was among those for whom mpox testing was retrospectively ordered by the clinician after negative diagnostic test results for other common rash illnesses, suggesting that clinician awareness was higher for mpox during this period. The data from the 2 analyses reported here indicate that as long as persons are aware of mpox and the need to seek medical care, the percentage of undiagnosed cases remains low, as it did during the peak of the outbreak.
The clinical manifestations (especially skin lesions, pustules, and rashes) of mpox patients can be confused with those of varicella zoster virus and STIs (e.g., herpes and syphilis), and mpox can co-occur with other STIs. However, in the molecular study, we did not find any significant levels of co-infections with mpox and other STIs.
That the earliest positive IgM result was obtained in mid-July suggests infection up to 56 days earlier. The lack of IgM detection before that time, in a small sample from 1 region, is suggestive that cases may not have been prevalent before the first detection on May 17. Of the 3 persons with an IgM-positive result, 2 self-reported symptoms consistent with mpox within the previous 3 months.
Among the limitations of our analyses, the response rates to the survey were low. The serologic survey relied on patient self-screening through the survey questionnaire, self-reported symptoms, and travel history. Also, the serologic survey was conducted in San Francisco, where infrastructure and resources may not be reflective of other geographic locations. Because the serologic survey was a point seroprevalence study, no follow-up testing or interviews were conducted among the participants who were positive for orthopoxvirus IgM; it is unknown whether any participants previously had signs/symptoms that were not reported on the survey or if signs/symptoms ultimately developed. Only 3 specimens were positive for both orthopoxvirus IgG and IgM. For the other 15 IgG-positive/IgM-negative specimens, it is unknown whether the participants had been exposed to orthopoxvirus beyond the IgM detection window or whether they did not self-report previous vaccination (many JYNNEOS vaccination campaigns were ongoing during the study period). We did not collect information on military service, which would include persons who may have received ACAM2000, a live-replicating vaccinia virus vaccine that results in production of orthopoxvirus antibodies. Because we used IgG as the initial screening tool, a participant could have been IgM positive and IgG negative; however, because that window of time is small (3–4 days), the likelihood of missing potential cases is low. The major limitations of molecular testing were similar to those of any study relying on ICD-10-CM codes for analysis and for which detailed patient history was not available beyond the ICD-10-CM codes on test requisitions.
In conclusion, the rate of undiagnosed mpox infections during the peak of reported cases in the United States was low among persons at high risk for disease (represented by participants in the San Francisco serosurvey). Mpox diagnosis was probably missed for some persons with rash (represented by retrospective molecular testing at HealthTrackRx), and providers should remain vigilant and conduct mpox testing from lesion swab samples on patients with mpox signs/symptoms. We rapidly collected our data during the peak of the outbreak to provide information for the epidemiologic response. Ongoing serologic and molecular studies that are underway that use specimens stored before May 2022 will be useful for determining whether mpox was present before the outbreak was identified in the United States.
Dr. Minhaj is an emergency medicine pharmacist/toxicologist and an epidemiologist at CDC within the Poxvirus and Rabies Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases. His work focuses on medical countermeasures related to orthopoxviruses.
Acknowledgments
We thank Inger Damon for early constructive conversations with HealthTrackRx, members of the CDC mpox outbreak response (including the Laboratory and Testing and Epidemiology Task Forces), Nathanael Gistand, staff at each participating clinic site, and the patients who volunteered for the serologic study. We also acknowledge Bernadette Aragon, Jon Oskarsson, and Judith Sansone for their research contributions.
Use of trade names and commercial sources are for identification only and do not imply endorsement by the US Department of Health and Human Services.
References
- Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al.; SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 countries. N Engl J Med. 2022;387:679–91. DOIPubMedGoogle Scholar
- Minhaj FS, Ogale YP, Whitehill F, Schultz J, Foote M, Davidson W, et al.; Monkeypox Response Team 2022. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764–9. DOIPubMedGoogle Scholar
- Hughes CM, Liu L, Davidson WB, Radford KW, Wilkins K, Monroe B, et al. A tale of two viruses: coinfections of monkeypox and varicella zoster virus in the Democratic Republic of Congo. Am J Trop Med Hyg. 2020;104:604–11. DOIPubMedGoogle Scholar
- Karem KL, Reynolds M, Braden Z, Lou G, Bernard N, Patton J, et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–72.PubMedGoogle Scholar
- Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121:1058–64. DOIPubMedGoogle Scholar
- Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. DOIPubMedGoogle Scholar
- Li Y, Zhao H, Wilkins K, Hughes C, Damon IK. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–7. DOIPubMedGoogle Scholar
- Dixon BE, Rahurkar S, Ho Y, Arno JN. Reliability of administrative data to identify sexually transmitted infections for population health: a systematic review. BMJ Health Care Inform. 2019;26:26. DOIPubMedGoogle Scholar
- Mauk KC, Torrone EA, Flagg EW. Can diagnostic codes in health care claims data identify confirmed chlamydial and gonococcal infections? A retrospective cohort study, 2003 to 2017. Sex Transm Dis. 2021;48(8S):S26–31. DOIPubMedGoogle Scholar
- Waddell CJ, Filardo TD, Prasad N, Pellegrini GJ Jr, Persad N, Carson WC, et al. Possible undetected mpox infection among persons accessing homeless services and staying in encampments—San Francisco, California. MMWR Morb Mortal Wkly Rep. 2023;72:227–31. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: October 13, 2023
1These authors contributed equally to this article.
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Faisal S. Minhaj, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H24-12, Atlanta, GA 30329-4027, USA
Top