Volume 29, Number 11—November 2023
Research Letter
Refractory Microascus Bronchopulmonary Infection Treated with Olorofim, France
Cite This Article
Citation for Media
Abstract
We report 3 cases of successful treatment of Microascus spp. bronchopulmonary infection in a multiple-traumatized patient and 2 lung transplant recipients in France. We emphasize the promising use of olorofim antifungal therapy in a rising context of intrinsically less-susceptible respiratory infections caused by mold.
The family Microascaceae includes genera Microascus and Scopulariopsis, opportunistic fungi that have caused respiratory infection associated with poor outcome and an attributable mortality rate of 85%–100% (1,2). Treatment of invasive Microascus infection is challenging because of its high resistance to available therapies. Olorofim, a reversible inhibitor of the enzyme dihyroorotate dehydrogenase, has shown in vitro activity against a variety of mold species, including azole-resistant Aspergillus (3,4) and Microascus spp. (5). We describe 3 cases of invasive Microascus respiratory infection in France that were treated with olorofim (Table). All patients gave informed consent for publication.
Case 1 occurred in a 17-year-old boy with unremarkable medical history who was found unconscious with inhalation pneumonia, bilateral hemopneumothorax, and bilateral thoracic drainage after falling from the top of a rice silo (Appendix Figure 1). On day 2, the patient underwent venovenous extracorporeal membrane oxygenation. On day 38, after 5 weeks of adapted antimicrobial treatment, thoracic computed tomography (CT) scan showed worsening of bilateral necrotizing pneumonia with abscess. Bronchoalveolar lavage (BAL) and several bronchial aspirations grew a restricted light-gray fungal colony (Appendix Figure 2), identified through the Paris National Reference Center as compatible with Microascus melanosporus; we initiated a combination of olorofim (180 mg 2×/d on day 1, followed by 90 mg 2×/d) and terbinafine (500 mg 2×/d) for 6 weeks (Appendix Table). Radiologic findings and general clinical status improved; we discontinued oxygen support after 2 weeks (day 73). The last CT scan showed complete healing of lung lesions (day 120). The patient was still alive 1 year later.
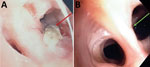
Case 2 occurred in a 61-year-old lung transplant recipient who sought care for respiratory deterioration and decline in respiratory function. He had recently received isavuconazole for bronchial colonization with Aspergillus flavus. Thoracic CT scan at admission showed a new alveolar consolidation in the left upper lobe (Appendix Figure 3); fibroscopy showed a recent-onset yellowish irregular lesion in the culminal bronchus (Figure, panel A). We isolated M. cirrosus from a culture of bronchial aspirate and BAL. We found no other disseminated lesions and retained the diagnosis of invasive pulmonary M. cirrosus infection. We initiated olorofim (90 mg 2×/d). We observed, as previously described (6), a moderate increase of both tacrolimus and everolimus blood through levels, which may have been caused by olorofim, a weak inhibitor of CYP 3A4. After 3 months of treatment, lung function slightly improved, CT scan showed a near-complete disappearance of the consolidation, and BAL culture was sterile. After 8 months of olorofim treatment, the endobronchial lesion was gone (Figure, panel B). M. cirrosus was found in BAL after 6 months of olorofim, but no more was cultured from BAL 7 months after treatment initiation. The patient was still being treated with olorofim at 9 months.
Case 3 occurred in a 65-year-old lung transplant recipient who sought care for dyspnea. He had experienced progressive decline of respiratory function and had a diagnosis of grade 3 bronchiolitis obliterative syndrome (BOS) linked to obstructive respiratory failure 6 years after transplant. He required permanent oxygen support. At admission, he received isavuconazole that continued for 3 months because of bronchial colonization with A. fumigatus. Thoracic CT scan results showed an unchanged pattern of BOS. Nevertheless, bronchial fibroscopy showed a new-onset bronchial lesion, necrotic and blackish in appearance, obstructing the origin of the culminal bronchus (Appendix Figure 4). We isolated M. cirrosus samples. Patient received a combination of oral terbinafine (500 mg 2×/d) and olorofim (180 mg 2×/d on day 1 followed by 90 mg 2×/d). After 3 months of treatment, bronchial fibroscopy showed an improvement of the bronchial lesion, and M. cirrosus was not found in respiratory specimens. The patient died from respiratory failure attributed to progression of BOS.
Use of olorofim for invasive Microascus spp. respiratory infection has not previously been reported with a successful outcome; previous studies were conducted in vitro (4). Miossec et al. (1) reported a series of 9 cases; all 9 patients had a medical history of stem cell or solid organ transplantation, and 8 died. The only survivor was a patient considered immunocompetent with no identified underlying conditions. A fatal Microascus sp. lung infection was previously published in a lung transplant recipient (6). Here, we report 2 lung transplant recipients infected with M. cirrosus, a ubiquitous mold isolated from soil and moist indoor environments (7). The third case we report was a young immunocompetent adult with no underlying conditions infected with M. melanosporus; his exposure by falling in a rice silo and sustaining serious injuries may explain the onset of opportunistic infection.
Microascus spp. and Scopulariopsis (8) exhibit a multidrug-resistant phenotype (9). Skóra et al. reported antifungal susceptibility results of several Microascus species and confirmed high resistance to ciclopirox, 5-fluorocytosine, amphotericin B, and azoles. However, among echinocandin, lower minimum effective concentrations for caspofungin were reported (10). The highest in vitro activity was observed with terbinafin (10); synergistic activity was observed against some Scopulariopsis strains (9). Wiederhold et al. reported promising activity of olorofim on Scopulariopsis spp. and Microascus spp. fungi (5), but no synergistic in vitro activity was reported between olorofim and terbinafine against Microascus spp.
Dr. Faure is an associate professor at Lille University and infectious disease specialist at Lille University Hospital. His primary interests include infection in immunocompromised hosts and host–pathogen interactions.
Acknowledgments
We thank F2G laboratory (Manchester, UK) for providing olorofim for compassionate use after reviewing the medical history of the cases with O.L. and F.L.
Author contributions: E.F., O.B., and E.C. were involved in patient care. C.C., P.C., and L.L. were mycologists in charge of laboratory investigations. O.L. and F.L. reviewed the Centre national de référence des mycoses invasives et antifongiques (CNRMA) materials. D.G.H. is responsible for mycological laboratory investigations in the CNRMA and performed identification of strains and extended antimicrobial susceptibility. E.H. managed the compassionate use in F2G laboratory. E.F., O.B., and F.L. wrote the manuscript. All co-authors reviewed the manuscript.
References
- Miossec C, Morio F, Lepoivre T, Le Pape P, Garcia-Hermoso D, Gay-Andrieu F, et al. Fatal invasive infection with fungemia due to Microascus cirrosus after heart and lung transplantation in a patient with cystic fibrosis. J Clin Microbiol. 2011;49:2743–7. DOIPubMedGoogle Scholar
- Liu Q, Kong L, Hua L, Xu S. Pulmonary Microascus cirrosus infection in an immunocompetent patient with bronchiectasis: a case report. Respir Med Case Rep. 2021;34:
101484 . DOIPubMedGoogle Scholar - Georgacopoulos O, Nunnally NS, Ransom EM, Law D, Birch M, Lockhart SR, et al. In vitro activity of novel antifungal olorofim against filamentous fungi and comparison to eight other antifungal agents. J Fungi (Basel). 2021;7:378. DOIPubMedGoogle Scholar
- Wiederhold NP. Review of the novel investigational antifungal olorofim. J Fungi (Basel). 2020;6:122. DOIPubMedGoogle Scholar
- Wiederhold NP, Patterson HP, Sanders CJ, Cañete-Gibas C. Dihydroorotate dehydrogenase inhibitor olorofim has potent in vitro activity against Microascus/Scopulariopsis, Rasamsonia, Penicillium and Talaromyces species. Mycoses. 2023;66:242–8. DOIPubMedGoogle Scholar
- Schoeppler KE, Zamora MR, Northcutt NM, Barber GR, O’Malley-Schroeder G, Lyu DM. Invasive Microascus trigonosporus species complex pulmonary infection in a lung transplant recipient. Case Rep Transplant. 2015;2015:
745638 . DOIPubMedGoogle Scholar - Woudenberg JHC, Meijer M, Houbraken J, Samson RA. Scopulariopsis and scopulariopsis-like species from indoor environments. Stud Mycol. 2017;88:1–35. DOIPubMedGoogle Scholar
- Aguilar C, Pujol I, Guarro J. In vitro antifungal susceptibilities of Scopulariopsis isolates. Antimicrob Agents Chemother. 1999;43:1520–2. DOIPubMedGoogle Scholar
- Cuenca-Estrella M, Gomez-Lopez A, Buitrago MJ, Mellado E, Garcia-Effron G, Rodriguez-Tudela JL. In vitro activities of 10 combinations of antifungal agents against the multiresistant pathogen Scopulariopsis brevicaulis. Antimicrob Agents Chemother. 2006;50:2248–50. DOIPubMedGoogle Scholar
- Skóra M, Bulanda M, Jagielski T. In vitro activities of a wide panel of antifungal drugs against various Scopulariopsis and Microascus species. Antimicrob Agents Chemother. 2015;59:5827–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: October 17, 2023
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Emmanuel Faure, CHRU de Lille—Service Universitaire de Maladies infectieuses, 1 rue Michel Polonovski Lille Nord 59037, France
Top