Volume 29, Number 12—December 2023
Dispatch
Treatment of Mpox with Suspected Tecovirimat Resistance in Immunocompromised Patient, United States, 2022
Figure 2
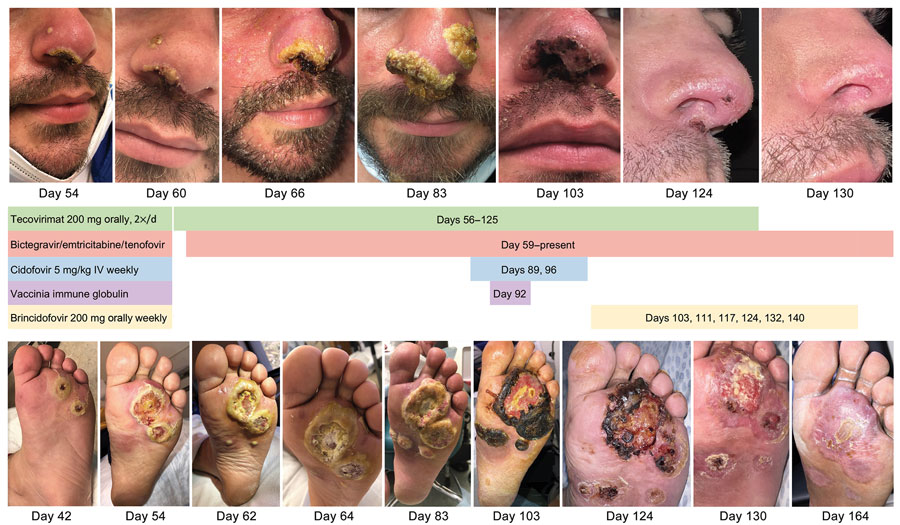
Figure 2. Visual timeline of facial lesions (top) and left plantar lesions (bottom) of immunocompromised patient with mpox, California, USA, 2022. Treatment received is indicated between images. IV, intravenous.
Page created: October 19, 2023
Page updated: November 18, 2023
Page reviewed: November 18, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.