Volume 29, Number 2—February 2023
Research
Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection
Figure 3
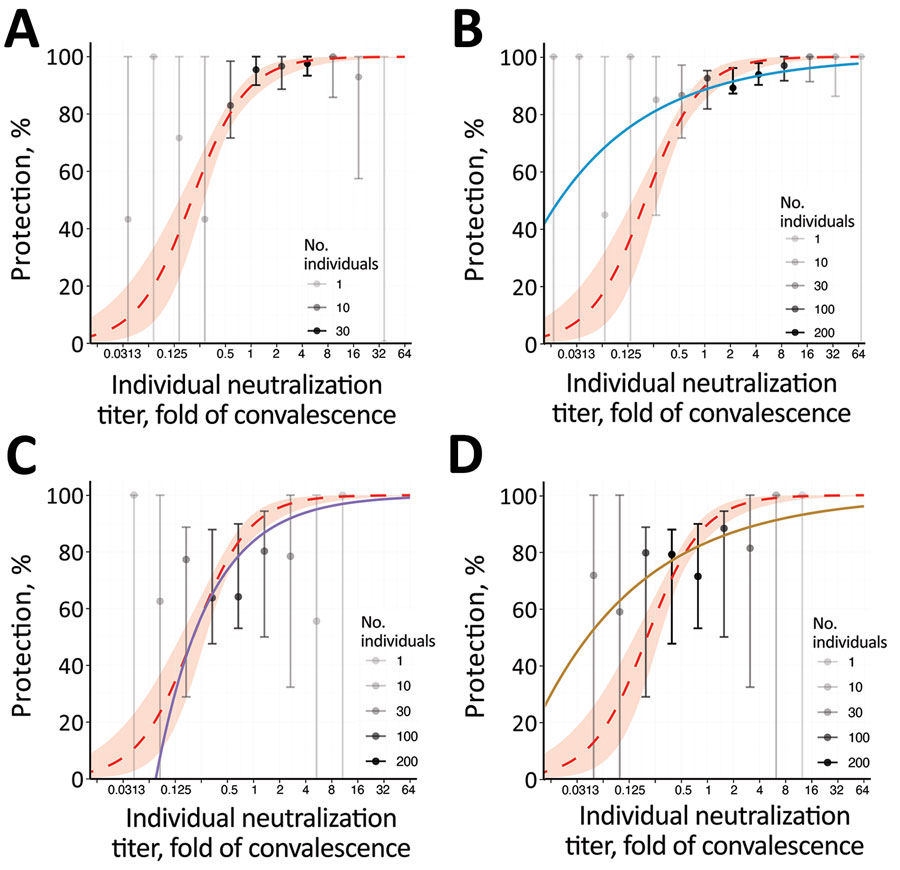
Figure 3. Breakthrough-infection data and protection from SARS-CoV-2 infection showing the association between neutralizing antibody titer and protection from symptomatic SARS-CoV-2 infection for an individual person. A) BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com) (5); B) mRNA-1273 (Moderna, https://www.modernatx.com), pseudovirus ID50 (4); C) ChAdOx1 (AstraZeneca, https://www.astrazeneca.com), live virus (3); D) ChAdOx 1, pseudovirus ID50 (3). The protection curve derived from the vaccine-comparison model (red dashed line and shading 95% CIs) is compared with the observed normalized frequencies of neutralization level (calculations in Appendix) of breakthrough infections reported in 3 studies (gray/black dots). Data from 2 mRNA vaccine studies of mRNA-1273 (A) and BNT162b2 (B), and the adenoviral vector vaccine ChAdOx1 nCoV19 (C, D) are shown. Lower opacity dots indicate fewer persons with neutralization titers in that range. Also shown in each panel are modelled protection curves showing the relationship between individual neutralizing antibodies and protection estimated in each breakthrough-infection study. Note: Breakthrough-infection data of BNT162b2 vaccinees were generously supplied by the authors of reference (5). The data were unavailable for the other 2 studies and were extracted from the original manuscripts; extraction of data from Gilbert et al. (4) was conducted manually and may be less reliable than that of the other studies (Appendix). ID50, 50% infectious dose; ID80, 80% infectious dose.
References
- Huddleston J, Barnes JR, Rowe T, Xu X, Kondor R, Wentworth DE, et al. Integrating genotypes and phenotypes improves long-term forecasts of seasonal influenza A/H3N2 evolution. eLife. 2020;9:
e60067 . DOIPubMedGoogle Scholar - Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. DOIPubMedGoogle Scholar
- Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al.; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–40. DOIPubMedGoogle Scholar
- Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al.; Immune Assays Team§; Moderna, Inc. Team§; Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) Team§; United States Government (USG)/CoVPN Biostatistics Team§. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. DOIPubMedGoogle Scholar
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–84. DOIPubMedGoogle Scholar
- Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–8. DOIPubMedGoogle Scholar
- World Health Organization. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. Geneva: Expert Committee on Biological Standardization; 2020. p. 9–10.
- Khoury DS, Wheatley AK, Ramuta MD, Reynaldi A, Cromer D, Subbarao K, et al. Measuring immunity to SARS-CoV-2 infection: comparing assays and animal models. Nat Rev Immunol. 2020;20:727–38. DOIPubMedGoogle Scholar
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al.; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. DOIPubMedGoogle Scholar
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al.; mRNA-1273 Study Group. mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–31. DOIPubMedGoogle Scholar
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al.; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. DOIPubMedGoogle Scholar
- Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–61. DOIPubMedGoogle Scholar
- Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al.; C4591007 Clinical Trial Group. C4591007 Clinical Trial Group. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. DOIPubMedGoogle Scholar
- Medicines & Healthcare Products Regulatory Agency; Access Consortium. alignment with ICMRA consensus on immunobridging for authorising new COVID-19 vaccines [cited 2022 Apr 8]. https://www.gov.uk/government/publications/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines
- Medicines & Healthcare Products Regulatory Agency. Guidance on strain changes in authorised COVID-19 vaccines [cited 2022 Apr 8]. https://www.gov.uk/government/publications/access-consortium-guidance-on-strain-changes-in-authorised-covid-19-vaccines/guidance-on-strain-changes-in-authorised-covid-19-vaccines
- Juno JA, Tan HX, Lee WS, Reynaldi A, Kelly HG, Wragg K, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020;26:1428–34. DOIPubMedGoogle Scholar
- Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12:1162. DOIPubMedGoogle Scholar
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al.; NGS-SA; COMMIT-KZN Team. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–6. DOIPubMedGoogle Scholar
- Cromer D, Reynaldi A, Steain M, Triccas JA, Davenport MP, Khoury DS. Relating in vitro neutralisation level and protection in the CVnCoV (CUREVAC) trial. Clin Infect Dis. 2022;75:e878–9. DOIPubMedGoogle Scholar
- Fong Y, McDermott AB, Benkeser D, Roels S, Stieh DJ, Vandebosch A, et al.; Immune Assays Team; the Coronavirus Vaccine Prevention Network (CoVPN)/ENSEMBLE Team; and the United States Government (USG)/CoVPN Biostatistics Team. Immune correlates analysis of the ENSEMBLE single Ad26.COV2.S dose vaccine efficacy clinical trial. Nat Microbiol. 2022;7:1996–2010. DOIPubMedGoogle Scholar
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–9. DOIPubMedGoogle Scholar
- O’Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al.; Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N Engl J Med. 2021;385:1184–95. DOIPubMedGoogle Scholar
- Cohen MS, Nirula A, Mulligan MJ, Novak RM, Marovich M, Yen C, et al.; BLAZE-2 Investigators. BLAZE-2 Investigators. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46–55. DOIPubMedGoogle Scholar