Volume 29, Number 5—May 2023
Research
Misdiagnosis of Clostridioides difficile Infections by Standard-of-Care Specimen Collection and Testing among Hospitalized Adults, Louisville, Kentucky, USA, 2019–20201
Figure 1
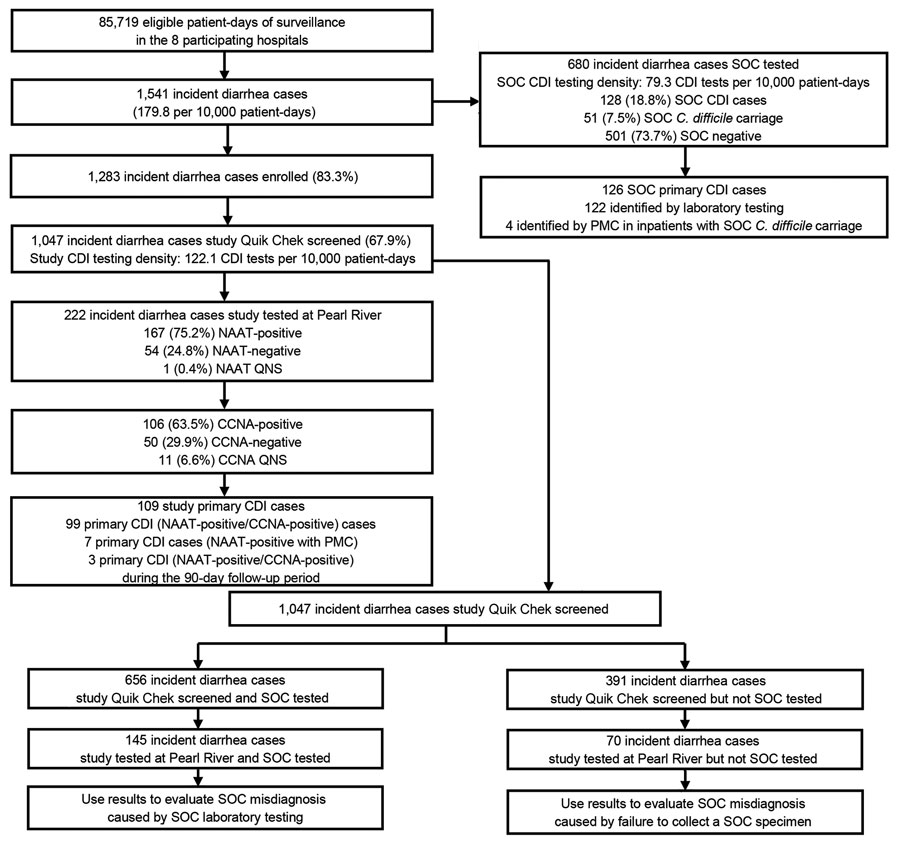
Figure 1. Incident diarrhea cases and testing of stool specimens among inpatients ≥50 years of age in Louisville, Kentucky, USA, before the COVID-19 pause in study of misdiagnosis of CDI by SOC specimen collection and testing among hospitalized adults, October 14, 2019–April 11, 2020. CCNA, cell culture cytotoxicity neutralization assay; CDI, Clostridioides difficile infection; NAAT, nucleic acid amplification test; PMC, pseudomembranous colitis; QNS, quantity not sufficient; Quik Chek, C. Diff Quik Chek Complete (Alere Techlab, https://www.techlab.com); SOC, standard-of-care.
1Study results were presented at the annual Anaerobe 2022 Congress, July 28–31, 2022, Seattle, Washington, USA.
2Current affiliation: Norton Healthcare, Louisville, Kentucky, USA.
3Current affiliation: AstraZeneca Pharmaceuticals, Wilmington, Delaware, USA.