Volume 29, Number 5—May 2023
Dispatch
Limited Nosocomial Transmission of Drug-Resistant Tuberculosis, Moldova
Abstract
Applying whole-genome-sequencing, we aimed to detect transmission events of multidrug-resistant/rifampin-resistant strains of Mycobacterium tuberculosis complex at a tuberculosis hospital in Chisinau, Moldova. We recorded ward, room, and bed information for each patient and monitored in-hospital transfers over 1 year. Detailed molecular and patient surveillance revealed only 2 nosocomial transmission events.
The main factor driving the epidemic of multidrug-resistant (MDR) and rifampin-resistant (RR) tuberculosis (TB) in Eastern Europe is active transmission of drug-resistant Mycobacterium tuberculosis complex (MTBC) (1). The role of nosocomial transmission of drug-resistant MTBC during prolonged hospitalizations remains poorly understood (2,3). We prospectively aimed to detect nosocomial transmission events at a TB referral hospital in Chisinau, the capital of Moldova.
We performed the study at the Chiril Draganiuc Phthisiopneumology Institute, Chisinau, Moldova. From July 1, 2014, through June 30, 2015, we prospectively tracked patients’ locations by room within the hospital, on the basis of the beds patients occupied each day during their hospital stays. We evaluated sputum samples by mycobacterial culture and performed phenotypic drug-susceptibility testing for all MTBC strains at admission. Sputum cultures for growth of MTBC were performed at least at the end of the second month, the fifth month, and the end of treatment in patients with drug-susceptible TB; for patients with MDR/RR TB, cultures were performed on a monthly basis until no growth of MTBC was detectable and quarterly thereafter (4). MTBC strains resistant to isoniazid and rifampin underwent whole-genome sequencing for genotypic prediction of drug resistance and phylogenetic comparison. All patients admitted to the study were followed up for 2 years after enrollment (Appendix 1). In total, 2,490 patients were admitted during the study period (Table 1; Appendix 1 Figure 1). The study was approved by the Research Ethical Committee of the State University of Medicine and Pharmacy (#15_49/2014), Chisinau, Moldova.
The number of patients with a confirmed diagnosis of TB by culture or the Xpert MTB/RIF assay (Cepheid, https://www.cepheid.com) was 1,016/1,379 (73.7%) (Table 1). Drug-susceptible strains of MTBC were found in 567/938 patients (60.5%), strains of mono/polydrug-resistant MTBC in 64/938 patients (6.8%), and strains of MDR/RR MTBC in 307/938 (32.7%) patients with detectable MTBC in culture. A total of 297/307 (96.7%) MDR/RR strains were available for analysis.
The median length of hospital stay was 22 days (interquartile range [IQR] 9–62 days) (Appendix 1 Figure 2, panel A). After admission, 75 patients were transferred to a different department than the one in which they were initially hospitalized (Appendix 1 Figure 2, panel B). Median length of stay until transfer to another department was 7 days (IQR 4–19 days) (Appendix 1 Figure 2, panel C).
A total of 41 patients with MDR/RR TB were initially admitted to a non–MDR TB departments. Of those, 33 patients were later transferred to the MDR TB department, and 8 patients were transferred to a different non–MDR TB department (Appendix 1 Figure 3, panel A). The median duration of stay for patients with MDR/RR TB in non–MDR TB departments was 7 days (IQR 4–18 days), and cumulative duration of stay was 631 days (Appendix 1 Figure 3, panel B). The median number of room-sharing contacts of patients with MDR/RR TB on non–MDR TB wards was 3 patients (IQR 2–5 patients), and the cumulative number of patients was 144.
A total of 17 patients (focus patients) with drug-susceptible MTBC strains at enrollment were found to be reinfected with an MDR/RR MTBC strain on follow-up. Only 1/144 roommates of the 41 patients with MDR/RR TB initially admitted to a non–MDR TB ward was in the same room with 1 of the 17 focus patients who potentially acquired MDR/RR TB on the non–MDR TB ward. For 297 patients with culture-confirmed MDR/RR TB at study enrollment and 17 focus patients with an MDR/RR MTBC reinfection detected during the follow-up period, 268 next-generation sequencing datasets were available for the molecular epidemiologic analysis (including all 17 strains from the focus patients).
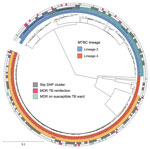
The bacterial population consisted of MTBC lineage 2 isolates (116/268, 43.3%) and lineage 4 isolates (152/268, 56.7%). Isolates in lineage 2 were typed as Central Asia L2-sublineage (67/116, 57.8%), Central Asia outbreak (21/116, 18.1%), and Europe/Russia W148 outbreak (25/116, 21.6%), whereas lineage 4 almost exclusively consisted of sublineage 4.2.1, URAL genotype (139/152, 91.4%) (Figure 1). We used sequence data to predict the resistance phenotype on the basis of direct association with previously described resistance-conferring single-nucleotide polymorphisms (SNPs) (Appendix 1 Figure 4).
To highlight putative transmission events between the focus patients and concurrently admitted patients with MDR/RR TB, we performed a molecular cluster analysis on the basis of pairwise genetic distance between all isolates (Figure 2). Overall, 124/268 (46.3%) patients were part of 1 of the 28 identified clusters, including 7/17 focus patients (Appendix 2 Table). Only 2/17 focus patients (sample ID 14 and ID 285) had a possible direct link in the hospital with a difference of <6 SNPs between the infecting MTBC strains, as well as 13 days and 29 days overlap in the hospital with their putative index case (Table 2; Appendix 1).
Nosocomial transmission of MTBC infection in high-burden settings has been reported previously (5–7). We prospectively aimed to detect transmission events of MDR/RR strains of MTBC at the TB referral center in Moldova, a country of high MDR/RR TB incidence. By matching each patient with a specific ward, room, and bed in the hospital for each day of the year, we were able to identify which of 307 patients with MDR/RR TB were initially wrongly allocated to a non–MDR TB ward, potentially leading to nosocomial transmission to other patients. Forty-one patients with MDR/RR TB initially spent a total of 631 days on non–MDR TB wards before drug-resistant TB was identified and they were transferred to an MDR TB ward. By using whole-genome sequencing on MDR/RR strains of MTBC from putative index patients and patients with drug-susceptible TB in whom MDR/RR TB then developed during follow-up, we identified only 2 highly likely transmission events, indicating a low rate of nosocomial transmission of MDR/RR strains of MTBC. Systematic implementation of basic infection control measures at the Chiril Draganiuc Phthisiopneumology Institute after previous indications of nosocomial transmission of MTBC (3) might have been effective in reducing TB transmission (8). However, these findings are limited by the high clonality of MTBC strains in patients with MDR/RR TB in Moldova (9), where more than one third of all incident TB cases are affected by multidrug/rifampin resistance (10). Our results call for further community efforts to reduce transmission of drug-resistant TB.
The first limitation of this study is that isolates were sampled only in the hospital, which could have introduced selection bias with persons without access to healthcare. However, because TB care in Moldova is centralized and provided free of charge, the effect of those factors should be minimal. Second, patients admitted to the hospital might have been subsequently readmitted to another hospital, in which case transmission events would have been missed. However, use of the national TB reporting database for follow-up minimizes the potential effect of this limitation. Third, the short time frame of this study might have missed transmission events, although most cases occur within 1 year of infection. Fourth, although a diagnostic delay occurred, transmission of MDR/RR MTBC could have been reduced already if patients had received empiric partly active treatment regimens. Finally, the rate of detected transmission might have been higher if transmission from patients with MDR/RR TB to other patients with MDR/RR TB had also been assessed.
In summary, in a detailed prospective evaluation at the TB referral hospital in Chisinau, Moldova, a high burden country of drug-resistant TB, we found that the rate of nosocomial transmission of MDR/RR strains of MTBC is low. Our results indicate the need for further community efforts to reduce transmission of drug-resistant strains of MTBC in high-burden settings.
Dr. Noroc is a scientific researcher at the National Tuberculosis Reference Laboratory of the Chiril Draganuic Tuberculosis Institute in Chisinau, Moldova, and a PhD candidate at the Research Center Borstel, Germany. Her primary research interests are genotypic and phenotypic methods for the diagnosis of tuberculosis and detecting transmission chains of drug-resistant strains of Mycobacterium tuberculosis by molecular methods.
Acknowledgment
D.C., M.M., I.B., V.D., and C.L. are supported by the German Center of Infection Research (DZIF).
References
- Brown TS, Eldholm V, Brynildsrud O, Osnes M, Levy N, Stimson J, et al. Evolution and emergence of multidrug-resistant Mycobacterium tuberculosis in Chisinau, Moldova. Microb Genom. 2021;7:
000620 . DOIPubMedGoogle Scholar - Genestet C, Paret R, Pichat C, Berland JL, Jacomo V, Carret G, et al.; Lyon TB study group. Routine survey of Mycobacterium tuberculosis isolates reveals nosocomial transmission. Eur Respir J. 2020;55:
1901888 . DOIPubMedGoogle Scholar - Crudu V, Merker M, Lange C, Noroc E, Romancenco E, Chesov D, et al. Nosocomial transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2015;19:1520–3. DOIPubMedGoogle Scholar
- Domente L, Alexandru S, Sain D, Nalivaico N, Ustian A, Moraru N, Rotaru-Lungu C. Protocol clinic naţional Tuberculoza la adult—PCN 123. Ministry of Health of the Republic of Moldova, Chisinau. 2012 [cited 2023 Apr 11]. https://ro.scribd.com/document/560260272/Pcn-123-Tb-Adult#
- Bantubani N, Kabera G, Connolly C, Rustomjee R, Reddy T, Cohen T, et al. High rates of potentially infectious tuberculosis and multidrug-resistant tuberculosis (MDR-TB) among hospital inpatients in KwaZulu Natal, South Africa indicate risk of nosocomial transmission. PLoS One. 2014;9:
e90868 . DOIPubMedGoogle Scholar - Gandhi NR, Weissman D, Moodley P, Ramathal M, Elson I, Kreiswirth BN, et al. Nosocomial transmission of extensively drug-resistant tuberculosis in a rural hospital in South Africa. J Infect Dis. 2013;207:9–17. DOIPubMedGoogle Scholar
- Smith JP, Modongo C, Moonan PK, Dima M, Matsiri O, Fane O, et al. Tuberculosis attributed to transmission within healthcare facilities, Botswana-The Kopanyo Study. Infect Control Hosp Epidemiol. 2022;43:1603–9. DOIPubMedGoogle Scholar
- Yang C, Sobkowiak B, Naidu V, Codreanu A, Ciobanu N, Gunasekera KS, et al. Phylogeography and transmission of M. tuberculosis in Moldova: A prospective genomic analysis. PLoS Med. 2022;19:
e1003933 . DOIPubMedGoogle Scholar - World Health Organization. Global tuberculosis report 2022. Geneva: The Organization; 2022.
Figures
Tables
Cite This ArticleOriginal Publication Date: April 14, 2023
1These first authors contributed equally to this article.
Table of Contents – Volume 29, Number 5—May 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Christoph Lange, Division of Clinical Infectious Diseases, Medical Clinic, Research Center Borstel, Parkallee 35, 23845 Borstel, Germany
Top