Volume 29, Number 6—June 2023
Dispatch
Novel Orthonairovirus Isolated from Ticks near China–North Korea Border
Cite This Article
Citation for Media
Abstract
We isolated a new orthonairovirus from Dermacentor silvarum ticks near the China–North Korea border. Phylogenetic analysis showed 71.9%–73.0% nucleic acid identity to the recently discovered Songling orthonairovirus, which causes febrile illness in humans. We recommend enhanced surveillance for infection by this new virus among humans and livestock.
Viruses of the genus Orthonairovirus, family Nairoviridae, include the consequential tick-transmitted pathogens Crimean–Congo hemorrhagic fever virus and Nairobi sheep disease virus, as well as other poorly characterized viruses that have been found in ticks and mammals. Orthonairovirus virions are spherical in shape (80–120-nm diameter) with 3 single-stranded RNA segments 17.1–22.8 kilobases in length and a membrane envelope (1–5). We performed surveillance in areas endemic for tick-borne encephalitis (6) and identified a novel orthonairovirus from Dermacentor silvarum ticks collected in 2021 in Jilin Province, China, near the China–North Korea border.
On April 17, 2021, we dragged corduroy to collect ticks from a forest region in Antu (118°46′E, 43°15′N), a district of the city of Yanbian in eastern Jilin Province, China, near the border with North Korea. We identified captured ticks according to morphologic keys and stored them at 4°C with wet cotton. We collected 264 ticks of 3 species—29 Ixodes persulcatus, 193 Dermacentor silvarum, and 12 Haemaphysalis concinna—and 30 larvae of unidentified species.
We homogenized ticks using a QIAGEN TissueLyser (QIAGEN, https://www.qiagen.com) and inoculated supernatants onto a monolayer of African green monkey kidney (Vero) E6 cells. After 3 successive passages, we observed cells for cytopathic effects. The inoculate from Dermacentor silvarum ticks, designated as YB_tick_2021_24, caused cytopathic effects in Vero E6 cells 96 h after inoculation (Figure 1, panels A, B). We collected cells showing cytopathic effects, then fixed and embedded them in epoxy resin. We cut ultrathin (80 nm) sections from the resin block, stained them with citrate lead and uranyl acetate, and observed them under a transmission electron microscope. We observed enveloped virus particles ≈100 nm in diameter that shared morphologic features with Bunyavirales viruses (Figure 1, panel C).
We extracted viral RNA from infected culture supernatants using a QIAGEN QIAamp Viral RNA Mini Kit, synthesized cDNA, prepared DNA libraries using an Illumina Nextera XT Kit (Illumina, https://www.illumina.com), and performed 150 bp paired-end sequencing using the Illumina MiniSeq System. We filtered reads on the basis of their length and mean quality values. We prepared contigs by de novo assembly and subjected them to BLASTx alignment (https://blast.ncbi.nlm.nih.gov/Blast.cgi) at E value <10−4 against the nonredundant protein and viral proteome databases of the National Center for Biotechnology Information. We used Bowtie 2 (https://bowtie-bio.sourceforge.net/bowtie2/index.shtml) to remap the clean reads to the generated virus-related contigs (7). We used rapid amplification of cDNA ends (RACE) PCR and Sanger sequencing to confirm the terminal sequences of virus genomes, and deposited the new genome in GenBank (accession nos. OQ207701–3). We identified open read frames (ORFs) using ORF finder (https://www.ncbi.nlm.nih.gov/orffinder) and calculated sequence similarities using BLAST.
Our procedure generated 40,826,350 reads (6.1 Gbp), which produced 266 virus-related contigs. Three contigs, the 1,516 bp small (S), 3,936 bp medium (M), and 12,133 bp large (L) segments, were annotated to Songling virus (SLV), a previously reported orthonairovirus (8). Average sequencing coverages remapped to the 3 contigs were 48× (S), 63× (M), and 234× (L). The final genome lengths confirmed by RACE sequencing were 1,848 bp encoding 488 aa for the S segment, 4,099 bp encoding 1,263 aa for the M segment, and 12,001 bp encoding 3,950 aa for the L segment. We performed multiple alignments using MAFFT version 7 (https://mafft.cbrc.jp/alignment/server) (9) and constructed a phylogenetic tree in MEGA7 (https://www.megasoftware.net) by using the neighbor-joining method with a bootstrap test for 1,000 replicates (10).
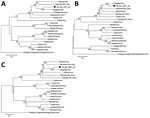
Phylogenetic analysis showed the strain belongs to the genus Orthonairovirus, family Nairoviridae, and is genetically related to SLV (Figure 2) (4,5,8,11). The terminal nucleotides of the S segment were identical to those of orthonairoviruses (3′ segment terminus AGAGUUUCU and 5′ segment terminus AGAAACUCU) (5). The termini of the M and L segments were different (Appendix Figure). Homology analysis comparing YB_tick_2021_24 with SLV sample YC585 showed 71.9% nucleic acid (na) and 71.5% aa identities for the S segment, 72.4% na and 79.5% aa identities for the M segment, and 73.0% na and 84.6% aa identities for the L segment (Table) (8). Those results indicate that the isolate represents a unique Orthonairovirus species. For purposes of archiving, we designated novel YB_tick_2021_24 as Antu virus and deposited the strain in the National Pathogen Resource Center (accession no. NPRC 2.3.9401).
We identified a novel orthonairovirus, Antu virus, in Dermacentor silvarum ticks collected in China near the China–North Korea border. Nucleotide and amino acid sequence homologies, combined with phylogenetic analysis of other orthonairovirus genomes, suggested that Antu virus is a new member of the genus Orthonairovirus, genetically related to SLV. Tamdy virus and SLV are orthonairoviruses reportedly able to infect human and livestock (8,12,13). Lacking direct evidence of the ability of Antu virus to infect and cause illness among humans and livestock animals, we recommend enhanced monitoring and surveillance for Antu virus infection among humans and livestock in potentially endemic areas.
Dr. Fan Li is an associate professor at National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Her research interests include virus discovery in disease vectors and arbovirus infections.
Acknowledgment
This study was supported by the Science and Technology Fundamental Resources Investigation Program (grant no. 2022FY100904), the National Key Research and Development Program (grant no. 2022YFC2302700, 2022YFC2602200), and the United States National Institutes of Health Foundation (grant no. U01 AI151810).
References
- Garrison AR, Alkhovsky Альховский Сергей Владимирович SV, Avšič-Županc T, Bente DA, Bergeron É, Burt F, et al. ICTV virus taxonomy profile: Nairoviridae. J Gen Virol. 2020;101:798–9. DOIPubMedGoogle Scholar
- Lasecka L, Baron MD. The molecular biology of nairoviruses, an emerging group of tick-borne arboviruses. Arch Virol. 2014;159:1249–65. DOIPubMedGoogle Scholar
- Bergeron É, Zivcec M, Chakrabarti AK, Nichol ST, Albariño CG, Spiropoulou CF. Recovery of recombinant Crimean Congo hemorrhagic fever virus reveals a function for non-structural glycoproteins cleavage by furin. PLoS Pathog. 2015;11:
e1004879 . DOIPubMedGoogle Scholar - Walker PJ, Widen SG, Wood TG, Guzman H, Tesh RB, Vasilakis N. A global genomic characterization of nairoviruses identifies nine discrete genogroups with distinctive structural characteristics and host-vector associations. Am J Trop Med Hyg. 2016;94:1107–22. DOIPubMedGoogle Scholar
- Kuhn JH, Wiley MR, Rodriguez SE, Bào Y, Prieto K, Travassos da Rosa AP, et al. Genomic characterization of the genus Nairovirus (family Bunyaviridae). Viruses. 2016;8:164. DOIPubMedGoogle Scholar
- Chen X, Li F, Yin Q, Liu W, Fu S, He Y, et al. Epidemiology of tick-borne encephalitis in China, 2007- 2018. PLoS One. 2019;14:
e0226712 . DOIPubMedGoogle Scholar - Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. DOIPubMedGoogle Scholar
- Ma J, Lv XL, Zhang X, Han SZ, Wang ZD, Li L, et al. Identification of a new orthonairovirus associated with human febrile illness in China. [Erratum in Nat Med. 2021;27:926.]. Nat Med. 2021;27:434–9. DOIPubMedGoogle Scholar
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. DOIPubMedGoogle Scholar
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. DOIPubMedGoogle Scholar
- Zhou H, Ma Z, Hu T, Bi Y, Mamuti A, Yu R, et al. Tamdy virus in Ixodid ticks infesting bactrian camels, Xinjiang, China, 2018. Emerg Infect Dis. 2019;25:2136–8. DOIPubMedGoogle Scholar
- Liu X, Zhang X, Wang Z, Dong Z, Xie S, Jiang M, et al. A tentative Tamdy orthonairovirus related to febrile illness in northwestern China. Clin Infect Dis. 2020;70:2155–60. DOIPubMedGoogle Scholar
- Moming A, Shen S, Fang Y, Zhang J, Zhang Y, Tang S, et al. Evidence of human exposure to Tamdy virus, northwest China. Emerg Infect Dis. 2021;27:3166–70. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: May 12, 2023
1These authors contributed equally to this article.
Table of Contents – Volume 29, Number 6—June 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Huanyu Wang, National Institute for Viral Disease Control and Prevention, State Key Laboratory for Infectious Disease Prevention and Control, Chinese Center for Disease Control and Prevention, No.155 Changbai Rd, Changping District, Beijing 102206, China
Top