Volume 30, Number 1—January 2024
Research
Effectiveness of Vaccines and Antiviral Drugs in Preventing Severe and Fatal COVID-19, Hong Kong
Figure 3
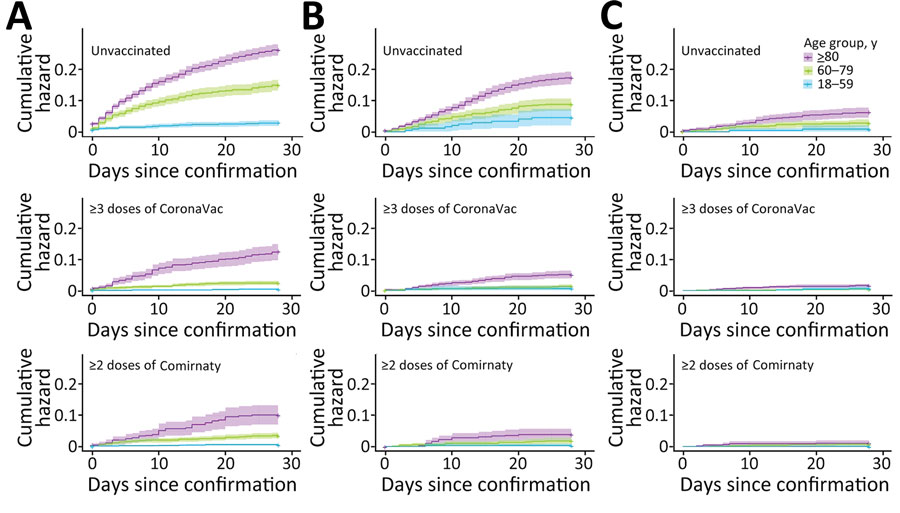
Figure 3. Cumulative hazards for all-cause mortality outcome events in study of effectiveness of vaccines and antiviral drugs in preventing severe and fatal COVID-19, Hong Kong. Cumulative hazards were compared among age groups, patients prescribed oral antiviral drugs, and those unvaccinated or vaccinated with CoronaVac or Comirnaty vaccines. A) No antiviral drugs, B) molnupiravir, C) nirmatrelvir/ritonavir. Antiviral drugs were prescribed within 5 days after confirmation of a COVID-19 diagnosis. Colors indicate age groups within each treatment group.
1These first authors contributed equally to this manuscript.
Page created: October 31, 2023
Page updated: December 20, 2023
Page reviewed: December 20, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.