Volume 30, Number 11—November 2024
Research Letter
Environmental Vibrio cholerae Strains Harboring Cholera Toxin and Vibrio Pathogenicity Island 1, Nigeria, 2008–2015
Cite This Article
Citation for Media
Abstract
Analysis of clinical and environmental Vibrio cholerae O1 strains obtained during 2008–2015 in Nigeria showed that lineages Afr9 and Afr12 carrying cholera toxin and Vibrio pathogenicity island 1 can be isolated from water. Our findings raise concerns about the role of the environment in maintenance and emergence of cholera outbreaks in Nigeria.
Nigeria is one of the current cholera hotspots in Africa (1). The World Health Organization report on cholera cases in countries in Africa for January 2022–December 2023 showed that most cases in West Africa were in Nigeria (n = 26,452) (2).
In 1970, the seventh cholera pandemic in Africa was initiated by the Vibrio cholerae O1 El Tor lineage (7PET), which became endemic to many countries in Africa (3). The pathogenicity of that lineage is characterized by 2 factors: cholera toxin, encoded by the ctxAB operon in the lysogenic bacteriophage CTXΦ, and the toxin coregulated pilus (TCP), encoded on the Vibrio pathogenicity island 1 and an essential factor for intestinal colonization and CTXΦ uptake (4). Weill et al. reconstructed the spatiotemporal spread of cholera in Africa during the seventh and current pandemics, showing that the 7PET lineage evolved into >13 sublineages and that the Afr9 and Afr12 lineages are the main sublineages causing cholera outbreaks in Nigeria and Cameroon (West Africa) (3). As part of efforts to provide information for cholera control, we used conventional microbiology, whole-genome sequencing, comparative genomics, and phylogenetic analysis to characterize clinical and environmental V. cholerae O1 strains obtained during the 2008–2015 cholera outbreaks in Nigeria.
We analyzed 24 V. cholerae strains comprising isolates from clinical (n = 16), environmental (n = 5), and unknown (n = 3) sources (Appendix). We used standard culture methods to identify and confirm that all strains were V. cholerae serogroup O1. We sequenced the genomes of those strains by using an Illumina Hiseq 2500 (https://www.illumina.com), assembled them with SPAdes v3.15.2 (https://github.com/ablab/spades), and analyzed them with Abricate by using the CARD and VFDB databases (https://github.com/tseemann/abricate). We analyzed the 24 environmental/clinical genomes from our study along with 36 other representative environmental/clinical V. cholerae genomes from Africa spanning all Afr sublineages (Afr1–12) of the seventh pandemic (3). We subjected genomes to a phylogenomic analysis that used Roary version 3.13.0 (https://github.com/sanger-pathogens/Roary), snp-dist version 2.5.1 (https://github.com/sanger-pathogens/snp-sites), and IQtree version 1.6.12 (https://github.com/Cibiv/IQ-TREE).
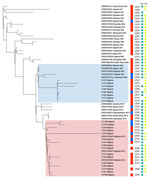
On the basis of the V. cholerae core genome, we determined that the 24 genomes from our study belonged to the Afr9 or Afr12 sublineages, including the clinical and environmental strains (Figure); those 2 sublineages have been associated with cholera outbreaks in countries in West Africa (3). The Afr9 genomes showed the wild-type sequence for GyrA and ParC, and the Afr12 genomes showed the S83I (GyrA) and S85L (ParC) mutations. V. cholerae strains from Nigeria had been previously characterized with those mutations, which were associated with resistance to nalidixic acid and decreased susceptibility to ciprofloxacin (5). The differences between the resistance profile of the Afr9 and Afr12 strains could be observed in the antimicrobial susceptibility profile (Table). Furthermore, we observed other resistance differences, mainly concerning resistance to streptomycin, sulfonamide, trimethoprim/sulfamethoxazole, and chloramphenicol (Table). By analyzing the resistome of the genomes (Appendix), we identified genes associated with resistance to those antimicrobials: aph(3′′)-Ib (strA) and aph(6)-Id (strB) (streptomycin), sul2 (sulfonamide), dfrA1 (trimethoprim/sulfamethoxazole), and floR (chloramphenicol). The genes were located in the integrative and conjugative element STX, which is predominant in genomes of current V. cholerae O1 strains, contrasting with 7PET strains from the 1970s (5). Of note, VC23, VC62, VC64 (Afr9), and VC105 (Afr12) presented a deletion in the integrative and conjugative element STX region that contained the strA/B, floR, and sul2 genes, which resulted in differences in the antimicrobial resistance profile between those strains and the others (Table).
Environmental and clinical genomes were related, particularly observed in 2 pairs of genomes: VC64 (Afr9/environmental/2007) and VC62 (Afr9/clinical/2007), and VC49 (Afr12/environmental/2010) and VC47 (Afr9/clinical/2010) (Figure). All Afr9 and Afr12 environmental genomes from Nigeria harbored the 2 major virulence determinants of epidemic V. cholerae O1, the ctxAB operon, and the TCP cluster, as well as most clinical genomes (Appendix). Those data represent evidence that strains belonging to the Afr9 and Afr12 epidemic lineages could be recovered from the environment in a West Africa country (Nigeria) and would still harbor the main virulence determinants of V. cholerae. A study conducted in East Africa (Tanzania) showed that the Afr10e sublineage, associated with a cholera outbreak in that region, could also be isolated from the environment (fish and water) and, as shown here, also harbored the ctxAB operon and the TCP cluster (8).
The global initiative for cholera control aims to reduce cholera deaths by 90% by 2030 (9). However, despite adoption of cholera elimination measures by many countries, cholera cases in 2023 demonstrated a huge and alarming resurgence across Africa, including Nigeria. The recent resurgence of cholera in some countries in Africa may be associated with climate change (10), but evidence of the presence of choleragenic Vibrio in the environment reveals the fundamental role of safe drinking water, sanitation, and hygiene in preventing and controlling cholera. Overall, our study highlights the need for continued genomic surveillance considering clinical and environmental V. cholerae strains.
Mr. Sergio is a postdoctoral fellow in bioinformatics at the Oswaldo Cruz Institute in Rio de Janeiro, Brazil. His primary research comprises genomic surveillance of environmental and clinical bacteria, also exploring their resistome, virulome, and mobilome.
Acknowledgments
The V. cholerae whole-genome sequences from this study were deposited in GenBank. Accession numbers are listed in the Appendix.
This study was financed by FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro), Processo SEI-260003/019688/2022.
A.C.V. was responsible for conceptualization, methodology, writing the original draft, review, editing, and funding acquisition. S.M. was responsible for methodology, formal analysis, writing the original draft, writing, review, and editing. É.F. was responsible for writing, review, and editing. F.F. was responsible for investigation. A.A was responsible for conceptualization, methodology, investigation, writing the original draft, and resources. I.A. was responsible for conceptualization, methodology, investigation, and writing the original draft. S.L. was responsible for conceptualization and resources.
References
- Charnley GEC, Jean K, Kelman I, Gaythorpe KAM, Murray KA. Association between conflict and cholera in Nigeria and the Democratic Republic of the Congo. Emerg Infect Dis. 2022;28:2472–81. DOIPubMedGoogle Scholar
- World Health Organization. Cholera in the WHO African Region: Weekly Regional Cholera Bulletin: 18 December 2023 [cited 2024 Mar 25]. https://iris.who.int/bitstream/handle/10665/375789/AFRO%20Cholera%20Bulletin.43.pdf
- Weill FX, Domman D, Njamkepo E, Tarr C, Rauzier J, Fawal N, et al. Genomic history of the seventh pandemic of cholera in Africa. Science. 2017;358:785–9. DOIPubMedGoogle Scholar
- Ramamurthy T, Mutreja A, Weill FX, Das B, Ghosh A, Nair GB. Revisiting the global epidemiology of cholera in conjunction with the genomics of Vibrio cholerae. Front Public Health. 2019;7:237. DOIPubMedGoogle Scholar
- Marin MA, Thompson CC, Freitas FS, Fonseca EL, Aboderin AO, Zailani SB, et al. Cholera outbreaks in Nigeria are associated with multidrug resistant atypical El Tor and non-O1/non-O139 Vibrio cholerae. PLoS Negl Trop Dis. 2013;7:
e2049 . DOIPubMedGoogle Scholar - Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline. 3rd ed. Austin (TX); The Institute; 2010.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 26th ed. Supplement M100S. Wayne (PA): The Institute; 2016.
- Hounmanou YMG, Njamkepo E, Rauzier J, Gallandat K, Jeandron A, Kamwiziku G, et al. Genomic Microevolution of Vibrio cholerae O1, Lake Tanganyika Basin, Africa. Emerg Infect Dis. 2023;29:149–53. DOIPubMedGoogle Scholar
- World Health Organization. Ending cholera: a global roadmap to 2030 [cited 2024 Mar 25]. https://www.gtfcc.org/wp-content/uploads/2019/10/gtfcc-ending-cholera-a-global-roadmap-to-2030.pdf
- Kaseya J, Dereje N, Tajudeen R, Ngongo AN, Ndembi N, Fallah MP. Climate change and malaria, dengue and cholera outbreaks in Africa: a call for concerted actions. BMJ Glob Health. 2024;9:
e015370 . DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: October 19, 2024
Table of Contents – Volume 30, Number 11—November 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sergio Morgado, Instituto Oswaldo Cruz, Av Brasil 4365, Rio de Janeiro 21040-360, Brazil
Top