Volume 30, Number 11—November 2024
Synopsis
Reemergence of Oropouche Virus in the Americas and Risk for Spread in the United States and Its Territories, 2024
Cite This Article
Citation for Media
Abstract
Oropouche virus has recently caused outbreaks in South America and the Caribbean, expanding into areas to which the virus was previously not endemic. This geographic range expansion, in conjunction with the identification of vertical transmission and reports of deaths, has raised concerns about the broader threat this virus represents to the Americas. We review information on Oropouche virus, factors influencing its spread, transmission risk in the United States, and current status of public health response tools. On the basis of available data, the risk for sustained local transmission in the continental United States is considered low because of differences in vector ecology and in human–vector interactions when compared with Oropouche virus–endemic areas. However, more information is needed about the drivers for the current outbreak to clarify the risk for further expansion of this virus. Timely detection and control of this emerging pathogen should be prioritized to mitigate disease burden and stop its spread.
Oropouche virus (genus Orthobunyavirus, Simbu serogroup) has recently been identified as a reemerging cause of widespread disease throughout the Americas (1). First discovered in Trinidad and Tobago in 1955, the virus caused periodic outbreaks of acute febrile illness in a limited number of countries in South and Central America for decades (2). Starting in late 2023, outbreaks of Oropouche virus disease were reported in areas with known endemic disease, and the virus emerged in new areas where it had not been historically documented. During January 1–September 6, 2024, more than 9,000 confirmed Oropouche virus disease cases and 2 deaths were reported from 6 countries: Bolivia, Brazil, Colombia, Cuba, the Dominican Republic, and Peru (1). In addition, several travel-associated cases have been reported among persons in the United States, Canada, and Europe traveling back from Cuba and Brazil (1,3,4). The recent expansion of the virus into previously nonendemic areas, identification of vertical transmission, and first reports of death from Oropouche virus disease have raised concerns about the broader threat this virus represents to the Americas, including the United States (1).
Oropouche virus circulates in both a sylvatic and an urban cycle. Sylvatic transmission, although not well understood, suggests a wide range of possible mammalian and avian hosts; virus has been detected in sloths (Bradypus tridactylus) and in several species of nonhuman primates, and antibodies have been found in domestic and wild birds and a rodent (5–7). Vectors hypothesized to be involved in sylvatic transmission include Aedes serratus and Coquillettidia venezuelensis mosquitoes (2,8). Humans develop sufficient viremia to contribute to viral spread, serving as bridge hosts that introduce Oropouche virus from its sylvatic maintenance cycle to populated areas. Once in the urban cycle, the virus circulates between humans and biting midges, Culicoides paraensis (9,10). The ubiquitous southern house mosquito, Culex quinquefasciatus, has also been suggested to play a role in urban transmission, although vector competency evaluations have shown mixed results (11–13) (Table; Figure 1).
Outbreaks of Oropouche virus have affected both urban and rural areas, and attack rates can be high; ≈30% of the population can be infected (17). Sex-specific attack rates have been inconsistent; some outbreaks disproportionately affect female persons and others affect more male persons (17,18). Some studies have shown that younger persons are more likely to be infected, possibly because of lack of previous exposure and immunity to the virus (17). A recent analysis of >5,000 confirmed cases identified in January 2015–March 2024 in Brazil showed approximately equal proportions of confirmed cases among male and female persons, and most reported infections occurred in persons 20–49 years of age (19). Those data suggest that persons with different demographic traits can be infected with Oropouche virus and that infection is driven by exposures, which might vary by sex, age, and daily activity (18).
The incubation period for Oropouche virus disease ranges from 3 to 10 days, and ≈60% of infected persons experience symptoms (8,20,21). Symptoms are similar to those of other vectorborne diseases, such as dengue, Zika, and chikungunya, and include acute onset of fever and severe headache, often with chills, myalgia, arthralgia, and fatigue. Other signs and symptoms can include photophobia, dizziness, retroorbital pain, nausea, vomiting, diarrhea, abdominal pain, conjunctival injection, and maculopapular rash (17,22,23). After the initial illness, up to 70% of persons can report relapse of symptoms, typically within a few days to weeks (6). Secondary episodes are clinically similar to the primary episode. No vaccines to prevent or medicines to treat Oropouche virus disease exist.
Although Oropouche virus disease is typically mild and reported deaths are rare, a small proportion of persons can develop more severe disease with hemorrhagic signs and symptoms (e.g., gingival bleeding, melena, and menorrhagia) or neurologic symptoms consistent with meningitis, meningoencephalitis, or Guillain-Barré syndrome (1,21,23,24). Of the 2 recent deaths associated with Oropouche virus among previously healthy young adult women, at least 1 patient had signs of hemorrhage (nasal, gingival, and vaginal bleeding and petechiae) starting 4 days after initial symptom onset (1). Neurologic symptoms have been reported in up to 4% of persons seeking clinical care (25). Signs and symptoms of neurologic disease can include occipital pain, dizziness, limb weakness, paresthesia, confusion, lethargy, photophobia, nausea, vomiting, nuchal rigidity, nystagmus, and paralysis (17,24,25).
In June 2024, vertical transmission of Oropouche virus was identified when RNA was detected in a stillborn infant born to a pregnant woman who had symptoms of Oropouche virus disease at 30 weeks’ gestation (1). After this identification, a retrospective investigation identified 4 infants with microcephaly in whom Oropouche virus IgM was detected in serum samples or cerebrospinal fluid (CSF) samples collected shortly after birth (26). In August 2024, an additional infant with microcephaly associated with Oropouche virus infection was reported. The infant, born in June 2024, tested positive for Oropouche virus IgM on the second day of life in serum and CSF. The infant later died at 47 days of life, and multiple tissues tested positive for Oropouche viral RNA (26). Further investigation is required to determine the frequency of vertical transmission and whether the timing of Oropouche virus disease during pregnancy increases the risk for an adverse outcome.
Testing for evidence of recent Oropouche virus infection can be performed on several different specimen types, though serum and CSF are used most often (27). During the first 7 days after infection, viral RNA can be detected through molecular testing such as reverse transcription PCR (RT-PCR). Most assays target the small (S) segment of the genome and cannot differentiate between Oropouche virus and other reassortant viruses (e.g., Iquitos virus) (27,28). After the first week of infection, antibody testing (e.g., IgM ELISA or plaque reduction neutralization test) is typically performed (29).
Viral RNA can be detected in the CSF of patients with neuroinvasive disease; however, it may not be present in the CSF at the time of clinical manifestation (because the virus is often cleared by that time), so serologic testing should be performed (25). Serologic testing is recommended for anyone experiencing a relapse of the disease because viral replication has not been detected during recurrence (8). Finally, in the event of fetal or infant death, postmortem tissues can be tested for evidence of antigen or viral RNA to assess causality (1).
The current outbreak in Latin America could be the result of lack of population-level immunity and viral reemergence in endemic areas, but other factors are possibly contributing to the spread and higher case counts. For example, changes to the viral genome through reassortment or vector distribution and competence might have resulted in more efficient transmission. Increased contact between humans and vectors caused by land use changes also could be contributing, because transmission activity has previously been detected in areas affected by deforestation (2). Finally, poor case recognition in the context of a large dengue outbreak could have furthered unchecked spread (i.e., because of lack of public health action when authorities are unaware of ongoing transmission).
Oropouche virus, like other orthobunyaviruses, is susceptible to reassortment, owing to its tripartite RNA genome, which includes the S segment encoding the nucleocapsid, medium (M) segment encoding the glycoproteins, and large (L) segment encoding L protein, which has RNA-directed RNA polymerase functions (30,31). The strain causing the current outbreak has shown some evidence of successive reassortment with genetically similar viruses (e.g., Perdões virus, Iquitos virus). Although the manner in which this strain might have influenced vector competence, disease severity, virus transmissibility, and immune protective status is not clear, preliminary research suggests reduced cross-neutralization with prototype strains in vitro (32). Reassortment has been observed with other orthobunyaviruses in the Americas (e.g., Fort Sherman virus, Potosi virus) and experimentally between Oropouche virus and orthobunyaviruses in the Simbu serogroup from outside the Americas (30,31,33).
Limited data exist regarding the specific vectors associated with recent urban outbreaks, although viral RNA has historically been detected in biting midges, including Cu. paraensis, and in Cx. quinquefasciatus mosquitoes (10,14). Cu. paraensis midges are found throughout the tropics, subtropics, and temperate areas in the Americas in wetland, forest, agricultural, rural, and periurban areas. In addition, Cx. quinquefasciatus mosquitoes are relatively ubiquitous, having a broad distribution in the northern and southern hemispheres. Temporally, outbreaks in Latin America have mostly coincided with the rainy season, during which biting midge and mosquito populations are typically more abundant (17,34).
Currently, large dengue outbreaks are occurring throughout the world; the Americas have reported unprecedented numbers of cases totaling >11 million since late 2023 (35). Because Oropouche virus disease and dengue have similar symptomology, they are difficult to distinguish clinically, and dengue testing is usually conducted before Oropouche virus testing is considered (29). This factor, combined with limited Oropouche testing availability, could have led to an underrecognition of increasing disease burden, which in turn might have led to a further expansion of outbreak and spread of the virus through infected persons into new areas.
As of September 2024, local transmission of Oropouche virus had not been reported in the United States, although some cases have been reported in travelers (4; https://www.cdc.gov/oropouche/data-maps.) Various factors are likely to affect the risk for local spread of the virus, including the rate of introduction from travel-associated cases, the presence and distribution of the vectors and potential host reservoirs, and potential virus adaptation.
Recent experiences with the introduction of chikungunya and Zika viruses to the United States could foretell what might occur with Oropouche virus, because all 3 arboviruses are maintained in an urban cycle between humans and arthropod vectors. During the chikungunya outbreak in 2014–2015, ≈3,700 travel-associated cases were reported in the continental United States. Despite thousands of possible introductions of viremic travelers, only 13 locally transmitted cases were identified in very limited areas of Florida and Texas (36). During the Zika virus outbreak in 2016–2017, US jurisdictions reported 5,389 travel-associated cases, resulting in 231 locally acquired cases, which also occurred in limited areas of Florida and Texas (37). Sustained local transmission of chikungunya and Zika was successfully thwarted by vector control and other public health interventions. Those experiences suggest that, even with frequent virus introductions through infected persons into the continental United States, large urban outbreaks of Oropouche are unlikely. For US territories, 4,900 locally acquired chikungunya cases were reported during 2014–2015 and 37,052 locally acquired cases of Zika virus were reported during 2016–2017 (36,37). Most of those cases were reported from Puerto Rico. On the basis of available data, the risk for sustained local transmission in the continental United States is likely low, whereas the risk for sustained transmission in Puerto Rico and the US Virgin Islands is unknown.
Most travel-associated Oropouche cases detected in Europe and the United States have been in travelers from Cuba (3,4). Cuba is in midst of its peak rainy season, which is associated with increased vector abundance (17,34), suggesting that more travel-associated cases might be expected from there. Previous research has not reported the primary vector of Cu. paraensis biting midges in Cuba, although Cx. quinquefasciatus mosquitoes and several biting midges of the Ceratopogonidae family have been detected there, including Cu. furens biting midges, which are also present in Florida (38). Vector competency evaluations have not been completed for many of those species, and a better understanding of transmission ecology in the Cuba outbreak and in the Dominican Republic will help to assess risk to the United States and, in particular, Puerto Rico.
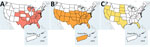
Both chikungunya and Zika viruses in the United States are transmitted by Aedes (Stegomyia) mosquitoes, which oviposit and develop in containers in and around homes, making persons more susceptible to mosquito exposure and, ultimately, infection. In contrast, the primary Oropouche vector, the Cu. paraensis biting midge, has low abundance in North America and mostly resides in tree holes in the southeast and midwestern United States (39–41) (Figure 2). In addition, the Cu. sonorensis biting midge is an another possible Oropouche vector, according to laboratory competency evaluations (15,16). Located mainly west of the Mississippi, this biting midge would be unlikely to perpetuate local Oropouche virus transmission in humans, because it is found in rural areas around livestock operations (15,42). Overall, taken together, the spatial distribution of biting midges in rural areas and poor vector competence in laboratory studies of mosquitoes translate to reduced risk for urban transmission in the United States, if Cu. paraensis biting midges are indeed the primary vector in ongoing Oropouche outbreaks.
Finally, despite its extreme abundance and enormous geographic range, the Cx. quinquefasciatus mosquito is not a very competent vector in laboratory studies and is the target of extensive West Nile virus vector control efforts (15,43,44). Existing control programs could therefore be adapted to the Oropouche context. On the other hand, Cx. quinquefasciatus mosquitoes have demonstrated widespread resistance to pyrethroids (particularly in parts of Florida), which could blunt the efficacy of vector control efforts. Cx. quinquefasciatus mosquitoes could represent a more serious threat to increase the risk for local transmission if it proves to be a competent vector. Of note, many mosquito (and Culicoides midge) species in the United States, which feed primarily on humans, have not been tested for vector competence of Oropouche virus.
Sylvatic transmission of Zika and chikungunya viruses has only been documented in Africa and relies on mosquitoes and nonhuman primates, whereas Oropouche virus maintenance in sylvatic settings can rely on wide array of species, on the basis of viral isolation and detection of antibodies in many different species (6). Oropouche virus has not been isolated or detected in birds, but Oropouche virus antibodies have been identified in >11 different families of wild and domestic birds in Brazil, raising questions about their role in transmission (5,14). Should the virus infect wild bird populations in North America, it is possible that Oropouche virus could become endemic, similar to the progression for West Nile virus. Oropouche virus’s propensity for reassortment could affect its ability to infect new hosts, enhance vector competence, and evade host immune response (45). However, the probability of sustained local transmission at this time is thought to be low in the continental United States because Oropouche virus would be required to overcome a series of biologic and ecologic obstacles.
In the past 25 years, the United States has experienced and responded to 4 different emergent mosquitoborne viral diseases, caused by West Nile, chikungunya, Zika, and dengue viruses. Given those experiences, preparation for potential Oropouche virus introductions into the United States could rely on several existing tools and interventions, including the current public health surveillance systems, case identification, vector control, personal protection, and public health communication.
ArboNET, the US national arboviral surveillance system, was established in 2000 in response to West Nile virus and can be adapted to capture data about new emerging and reemerging arboviruses (https://www.cdc.gov/oropouche/data-maps/current-year-data.html). ArboNET enables reporting of human disease cases, human infections (e.g., presumptive viremic donors), animal disease, sentinel animal infections, and vector infections. Human disease cases are reported from state and territorial health departments using standard case definitions. Case reports can include information on travel location, clinical manifestations, and transmission mechanisms (46).
Oropouche virus disease is not a nationally notifiable condition, but state and territorial health departments can voluntarily report identified cases to ArboNET. In addition, if Oropouche virus emerges in the United States, the Council of State and Territorial Epidemiologists can decide whether to make Oropouche virus disease nationally notifiable and determine whether a new case definition should be developed to capture potential fetal deaths or congenital infections, as was done for Zika virus previously (47).
Clinicians should report suspected Oropouche virus disease cases to state or local health departments to enable testing and to implement community prevention measures and messaging. Information about clinical features, diagnosis, and clinical management is available on the Centers for Disease Control and Prevention (CDC) website (https://www.cdc.gov/oropouche/hcp/clinical-overview). At this time, testing for Oropouche virus should be considered when a patient has traveled within 2 weeks of initial symptom onset (because patients can experience recurrent symptoms) to an area with documented or suspected Oropouche virus circulation and has an abrupt onset of fever, headache, and >1 of the following signs/symptoms: myalgia, arthralgia, photophobia, retroorbital/eye pain, or indications of neuroinvasive disease (e.g., stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis). If concern exists for local transmission in a nonendemic area, providers should consider whether the patient had contact with a person with confirmed Oropouche virus infection, lives in an area where travel-related cases have been identified, or has known vector exposure (e.g., mosquitoes or biting midges). In addition, testing should only be considered among patients who tested negative for other pathogens, in particular dengue. If strong suspicion of Oropouche virus disease exists on the basis of the patient’s clinical features and history of travel to an area with virus circulation, providers should not wait on negative test results before sending specimens to CDC. This guidance on clinical case identification will likely need to be modified as the epidemiologic situation evolves, including whether local transmission is identified, and as more is learned about clinical manifestations and transmission risk, including for vertical transmission and potential adverse birth outcomes.
Available vector control tools, such as insecticide spraying and source reduction (modification of larval habitats to prevent oviposition), are similar for biting midges and mosquitoes, but questions remain about the application of such tools in the context of Oropouche. Empirical evaluations of Cu. paraensis midge–specific control measures are lacking. Previous works have shown aerial spraying of the insecticide naled has resulted in substantial reduction (up to 99%) in pestiferous Culicoides species (48). Source reduction around dairy operations for Cu. sonorensis midges and removal of leaf waste for Cu. paraensis midges have also been used, with varying degrees of success (49). Cx. quinquefasciatus mosquitoes are abundant and widely distributed; therefore, control activities should be determined on the basis of mosquito surveillance data to more efficiently target when and where this species is active. A combination of larviciding and adulticiding will be most useful, given the asynchronous hatching of this mosquito’s egg rafts. Challenges in implementing vector control include the limited scope of vector control agencies that primarily target mosquitoes and are not mandated to manage other arthropods, lack of acceptability of aerial spraying of insecticides, insecticide resistance, and limited utility of larval source reduction because of the cryptic nature of some larval habitats (i.e., tree holes for Cu. paraensis midges) (39).
Persons can protect themselves against both midge and mosquito bites by wearing long sleeves and pants and by using an insect repellant registered by the US Environmental Protection Agency. Those products are safe for pregnant and breastfeeding women when used as directed; for children <3 years of age, products containing oil of lemon eucalyptus or para-menthane-diol should not be used. Windows and door screens can also prevent mosquitoes from entering the home and protect against vectorborne diseases (50). However, Culicoides spp. midges are smaller than typical window screen holes and can pass through and enter the home. Mesh size 20 (or 20 × 20 mesh, which has 20 openings in 1 linear inch) is designed to exclude biting midges. Patients with suspected Oropouche virus disease should avoid being bitten by biting midges and mosquitoes for 1 week to prevent infection of naive vectors.
Strong engagement with the community is necessary to gain support for vector control activities, as well as to improve the uptake of personal protective measures, which are currently the only ways to prevent infection. Rapidly distributing information to public health professionals, providers, the public, and other stakeholders can lead to improved surveillance, diagnosis, and implementation of prevention strategies. The use of various platforms for distribution of communications (e.g., CDC website, Health Alert Network messages, social media posts, publications, and data dashboards) can improve the reach and distribution of messages about Oropouche virus and ways persons can prevent themselves from being infected and spreading the virus.
Overall, on the basis of current knowledge, the risk for localized outbreaks of Oropouche virus disease in most areas in the United States should be considered low because of differences in vectors and human-vector interactions (e.g., mitigation by widespread availability of closeable windows and air-conditioning) compared with endemic areas. Some states and territories are probably at elevated risk for local spread, including those where infected travelers are most likely to arrive and be readily exposed to vectors, such as southern Florida or Puerto Rico. Past experiences with several emerging and reemerging vectorborne diseases, as well as new information from Oropouche outbreaks (e.g., transmission ecology in Cuba), will help to inform and refine preparedness, detection, and response to Oropouche virus. Public health partners should prioritize timely detection and control of this emerging pathogen to prevent human disease cases and the spread of the virus.
Dr. Guagliardo is an epidemiologist with the Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention in Fort Collins, Colorado. She has a longstanding interest in the epidemiology and ecology of vectorborne and zoonotic diseases.
Acknowledgment
We thank Roberto Barrera and Saul Lozano for their assistance with understanding geographic distribution of vectors.
References
- Pan American Health Organization/World Health Organization. Epidemiological alerts and updates. [cited 2024 Sep 23]. https://www.paho.org/en/epidemiological-alerts-and-updates
- Anderson CR, Spence L, Downs WG, Aitken TH. Oropouche virus: a new human disease agent from Trinidad, West Indies. Am J Trop Med Hyg. 1961;10:574–8. DOIPubMedGoogle Scholar
- European Centers for Disease Prevention and Control. Threat assessment brief: Oropouche virus disease cases imported into the European Union, 9 August 2024 [cited 2024 Sep 23]. https://www.ecdc.europa.eu/sites/default/files/documents/TAB-Oropouche-august-2024.pdf
- Morrison A, White JL, Hughes HR, Guagliardo SAJ, Velez JO, Fitzpatrick KA, et al. Oropouche virus disease among U.S. travelers—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73:769–73. DOIPubMedGoogle Scholar
- Pinheiro FP, Travassos da Rosa AP, Travassos da Rosa JF, Bensabath G. An outbreak of Oropouche virus diease in the vicinity of santarem, para, barzil. Tropenmed Parasitol. 1976;27:213–23.PubMedGoogle Scholar
- Azevedo RS, Nunes MR, Chiang JO, Bensabath G, Vasconcelos HB, Pinto AY, et al. Reemergence of Oropouche fever, northern Brazil. Emerg Infect Dis. 2007;13:912–5. DOIPubMedGoogle Scholar
- Nunes MR, Martins LC, Rodrigues SG, Chiang JO, Azevedo RS, da Rosa AP, et al. Oropouche virus isolation, southeast Brazil. Emerg Infect Dis. 2005;11:1610–3. DOIPubMedGoogle Scholar
- Pinheiro FP, Travassos da Rosa AP, Travassos da Rosa JF, Ishak R, Freitas RB, Gomes ML, et al. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am J Trop Med Hyg. 1981;30:149–60. DOIPubMedGoogle Scholar
- Pinheiro FP, Travassos da Rosa AP, Gomes ML, LeDuc JW, Hoch AL. Transmission of Oropouche virus from man to hamster by the midge Culicoides paraensis. Science. 1982;215:1251–3. DOIPubMedGoogle Scholar
- Roberts DR, Hoch AL, Dixon KE, Llewellyn CH. Oropouche virus. III. Entomological observations from three epidemics in Pará, Brazil, 1975. Am J Trop Med Hyg. 1981;30:165–71. DOIPubMedGoogle Scholar
- Cardoso BF, Serra OP, Heinen LB, Zuchi N, Souza VC, Naveca FG, et al. Detection of Oropouche virus segment S in patients and inCulex quinquefasciatus in the state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2015;110:745–54. DOIPubMedGoogle Scholar
- Hoch AL, Pinheiro FP, Roberts DR, Gomes ML. Laboratory transmission of Oropouche virus by Culex quinquefasciatus Say. Bull Pan Am Health Organ. 1987;21:55–61.PubMedGoogle Scholar
- de Mendonça SF, Rocha MN, Ferreira FV, Leite THJF, Amadou SCG, Sucupira PHF, et al. Evaluation of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes competence to Oropouche virus infection. Viruses. 2021;13:755. DOIPubMedGoogle Scholar
- LeDuc JW, Hoch AL, Pinheiro FP, da Rosa AP. Epidemic Oropouche virus disease in northern Brazil. Bull Pan Am Health Organ. 1981;15:97–103.PubMedGoogle Scholar
- McGregor BL, Connelly CR, Kenney JL. Infection, dissemination, and transmission potential of North American Culex quinquefasciatus, Culex tarsalis, and Culicoides sonorensis for Oropouche virus. Viruses. 2021;13:226. DOIPubMedGoogle Scholar
- McGregor BL, Shults PT, McDermott EG. A review of the vector status of North American Culicoides (Diptera: Ceratopogonidae) for bluetongue virus, epizootic hemorrhagic disease virus, and other arboviruses of concern. Curr Trop Med Rep. 2022;9:130–9. DOIPubMedGoogle Scholar
- Pinheiro FP, Travassos da Rosa APA, Vasconcelos PF. Oropouche fever. In: Feigin RD, Demmler GJ, Cherry, JD, Kaplan SL, editors. Textbook of pediatric infectious diseases. Philadelphia: Elsevier; 2004. p. 2418–23.
- Baisley KJ, Watts DM, Munstermann LE, Wilson ML. Epidemiology of endemic Oropouche virus transmission in upper Amazonian Peru. Am J Trop Med Hyg. 1998;59:710–6. DOIPubMedGoogle Scholar
- Martins-Filho PR, Carvalho TA, Dos Santos CA. Spatiotemporal epidemiology of Oropouche fever, Brazil, 2015–2024. Emerg Infect Dis. 2024;30:2196–8. DOIPubMedGoogle Scholar
- Freitas RB, Pinheiro FP, Santos MAV, Travassos da Rodsa APA, Travassos da Rosa JFS, Nazareno de Freitas E. Oropouche virus epidemic in the eastern state of Para, 1979 [in Portuguese]. Rev Fundacao Servicos Saude Publica. 1980;25:59–72.
- Vernal S, Martini CCR, da Fonseca BAL. Oropouche virus–associated aseptic meningoencephalitis, southeastern Brazil. Emerg Infect Dis. 2019;25:380–2. DOIPubMedGoogle Scholar
- Vasconcelos PF, Travassos Da Rosa JF, Guerreiro SC, Dégallier N, Travassos Da Rosa ES, Travassos Da Rosa AP. [1st register of an epidemic caused by Oropouche virus in the states of Maranhão and Goiás, Brazil] [in Portuguese]. Rev Inst Med Trop São Paulo. 1989;31:271–8. DOIPubMedGoogle Scholar
- Mourãão MP, Bastos MS, Gimaqu JB, Mota BR, Souza GS, Grimmer GH, et al. Oropouche fever outbreak, Manaus, Brazil, 2007-2008. Emerg Infect Dis. 2009;15:2063–4. DOIPubMedGoogle Scholar
- de Armas Fernández JR, Peña García CE, Acosta Herrera B, Betancourt Plaza I, Gutiérrez de la Cruz Y, Resik Aguirre S, et al. Report of an unusual association of Oropouche Fever with Guillain-Barré syndrome in Cuba, 2024. Eur J Clin Microbiol Infect Dis. 2024; Epub ahead of print. DOIPubMedGoogle Scholar
- Pinheiro FP, Rocha AG, Freitas RB, Ohana BA, Travassos da Rosa AP, Rogério JS, et al. [Meningitis associated with Oropouche virus infections] [in Portuguese]. Rev Inst Med Trop Sao Paulo. 1982;24:246–51.PubMedGoogle Scholar
- Ministry of Health Brazil, Secretariat of Health and Environment Surveillance. Joint technical note no. 135/2024-SVSA/SAPS/SAES/MS [in Portuguese] [cited 2024 Aug 14]. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/notas-tecnicas/2024/nota-tecnica-conjunta-no-135-2024-svsa-saps-saes-ms
- Moreli ML, Aquino VH, Cruz AC, Figueiredo LT. Diagnosis of Oropouche virus infection by RT-nested-PCR. J Med Virol. 2002;66:139–42. DOIPubMedGoogle Scholar
- Naveca FG, Nascimento VAD, Souza VC, Nunes BTD, Rodrigues DSG, Vasconcelos PFDC. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and Oropouche-like viruses. Mem Inst Oswaldo Cruz. 2017;112:510–3. DOIPubMedGoogle Scholar
- Pan American Health Organization/World Health Organization. Guidelines for the detection and surveillance of emerging arboviruses in the context of the circulation of other arboviruses [cited 2024 Sep 23]. https://www.paho.org/en/documents/guidelines-detection-and-surveillance-emerging-arboviruses-context-circulation-other
- Briese T, Calisher CH, Higgs S. Viruses of the family Bunyaviridae: are all available isolates reassortants? Virology. 2013;446:207–16. DOIPubMedGoogle Scholar
- Tilston-Lunel NL, Shi X, Elliott RM, Acrani GO. The potential for reassortment between Oropouche and Schmallenberg orthobunyaviruses. Viruses. 2017;9:220. DOIPubMedGoogle Scholar
- Naveca FG, de Almeida TAP, Souza V, Nascimento V, Silva D, Nascimento F, et al. Human outbreaks of a novel reassortant Oropouche virus in the Brazilian Amazon region. Nat Med. 2024; Epub ahead of print. DOIPubMedGoogle Scholar
- Briese T, Kapoor V, Lipkin WI. Natural M-segment reassortment in Potosi and Main Drain viruses: implications for the evolution of orthobunyaviruses. Arch Virol. 2007;152:2237–47. DOIPubMedGoogle Scholar
- Feitoza LHM, de Carvalho LPC, da Silva LR, Meireles ACA, Rios FGF, Silva GS, et al. Influence of meteorological and seasonal parameters on the activity of Culicoides paraensis (Diptera: Ceratopogonidae), an annoying anthropophilic biting midge and putative vector of Oropouche Virus in Rondônia, Brazilian Amazon. Acta Trop. 2023;243:
106928 . DOIPubMedGoogle Scholar - Pan American Health Organization/World Health Organization. Dengue [cited 2024 Sep 23]. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en.html
- Centers for Disease Control and Prevention. Chikungunya in the United States [cited 2024 Sep 23]. https://www.cdc.gov/chikungunya/data-maps/chikungunya-us.html
- Centers for Diseases Control and Prevention. Zika cases in the United States [cited 2024 Sep 23]. https://www.cdc.gov/zika/zika-cases-us/index.html
- Borkent AD, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:001–377.
- Pappas LG, Moyer S, Pappas CD. Tree hole Culicoides (Diptera: Ceratopogonidae) of the Central Plains in the United States. J Am Mosq Control Assoc. 1991;7:624–7.PubMedGoogle Scholar
- Wirth WW, Dyce AL, Peterson BV. An atlas of wing photographs, with a summary of the numerical characters of the Nearctic species of Culicoides (Diptera: Ceratopogonidae). Contrib Am Entomol Inst. 1985;22:1–46.
- Integrated Digitalized Biocollections (iDigBio). Specimen record: Culicoides paraensis [cited 2024 Sep 23]. https://www.idigbio.org/portal/records/b63faf1c-dd9f-4053-b600-001617263c5e
- Schmidtmann ET, Herrero MV, Green AL, Dargatz DA, Rodriquez JM, Walton TE. Distribution of Culicoides sonorensis (Diptera: Ceratopogonidae) in Nebraska, South Dakota, and North Dakota: clarifying the epidemiology of bluetongue disease in the northern Great Plains region of the United States. J Med Entomol. 2011;48:634–43. DOIPubMedGoogle Scholar
- Gorris ME, Bartlow AW, Temple SD, Romero-Alvarez D, Shutt DP, Fair JM, et al. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit Vectors. 2021;14:547. DOIPubMedGoogle Scholar
- Darcie RFJ, Ward RA. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. Gainesville (FL): University Press of Florida; 2005.
- Vijaykrishna D, Mukerji R, Smith GJ. RNA virus reassortment: an evolutionary mechanism for host jumps and immune evasion. PLoS Pathog. 2015;11:
e1004902 . DOIPubMedGoogle Scholar - Centers for Disease Control and Prevention. Arboviral diseases, neuroinvasive and non-neuroinvasive: 2015 case definition [cited 2024 Sep 23]. https://ndc.services.cdc.gov/case-definitions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive-2015
- Council of State and Territorial Epidemiologists. Zika virus disease and Zika virus infection without disease, including congenital infections case definitions and addition to the nationally notifiable diseases list [cited 2024 Sep 23]. https://cdn.ymaws.com/www.cste.org/resource/resmgr/2016PS/16_ID_01_edited7.29.pdf
- Breidenbaugh MS, de Szalay FA. Effects of aerial applications of naled on nontarget insects at Parris Island, South Carolina. Environ Entomol. 2010;39:591–9. DOIPubMedGoogle Scholar
- Purse BV, Carpenter S, Venter GJ, Bellis G, Mullens BA. Bionomics of temperate and tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu Rev Entomol. 2015;60:373–92. DOIPubMedGoogle Scholar
- Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9:86–9. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: October 01, 2024
Table of Contents – Volume 30, Number 11—November 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sarah Anne J. Guagliardo, Centers for Disease Control and Prevention, 3156 Rampart Rd, Fort Collins, CO 80521, USA
Top