Volume 30, Number 12—December 2024
Synopsis
Bartonella quintana Endocarditis in Persons Experiencing Homelessness, New York, New York, USA, 2020–2023
Cite This Article
Citation for Media
Abstract
Bartonella quintana infection can lead to bacillary angiomatosis, peliosis hepatis, chronic bacteremia, and culture-negative endocarditis. Transmitted by the human body louse (Pediculus humanus humanus), B. quintana infection has become an emerging disease in recent decades among persons experiencing homelessness. By using retrospective laboratory surveillance, we identified 5 cases of left-sided, culture-negative B. quintana endocarditis among persons in New York, New York, USA, during January 1, 2020–November 23, 2023. Identifications were made by using molecular assays. All patients experienced unsheltered homelessness in the year before hospitalization. Of those patients, 4 experienced heart failure, 3 renal failure, and 2 embolic strokes; 2 died. Aortic valve replacement occurred in 4 cases. A history of possible body louse infestation was found in 4 cases. Clinicians should consider housing status and history of lice exposure in patients with suspected bartonellosis and have a low threshold for diagnostic testing and empiric treatment in patients experiencing homelessness.
Bartonella bacteria are fastidious, intracellular gram-negative rods that cause disease in humans, cats, dogs, rodents, and other mammals. Thirteen Bartonella species are known to cause human infection (1). B. quintana infection can cause bacillary angiomatosis, peliosis hepatis, chronic bacteremia (2–4), and culture-negative endocarditis (5,6). B. quintana is transmitted by the human body louse (Pediculus humanus humanus) through inoculation of broken skin by contaminated feces. Infection is enabled in settings where personal hygiene is compromised, such as refugee camps (7) and wartime environments (8). In recent decades, B. quintana infection has emerged as a disease primarily affecting persons experiencing homelessness (3,5,9–20).
In January 2023, B. quintana infection was diagnosed in 2 kidney transplant recipients from 1 donor (21). An investigation by the New York City (NYC) Department of Health and Mental Hygiene (New York, NY, USA) determined the donor had experienced unsheltered homelessness and had B. quintana bacteremia during their terminal hospitalization in summer 2022. In April 2023, another case of Bartonella endocarditis diagnosed in a patient experiencing homelessness was reported to the NYC Department of Health and Mental Hygiene, prompting efforts to identify additional cases to better understand the incidence and severity of B. quintana infections in the city (22). This article provides information regarding the clinical manifestations, diagnosis, and treatment of each case and discusses management options for clinicians caring for patients experiencing homelessness who might have bartonellosis.
The NYC Department of Health and Mental Hygiene conducted retrospective active surveillance for patients with a diagnosed B. quintana infection among clinical laboratories of 5 large hospital networks and the Wadsworth Center at the New York State Department of Health. The laboratories identified patients during January 1, 2020–November 23, 2023, who had a Bartonella spp. recovered from specimen cultures; who tested positive for Bartonella spp. with PCR further confirmed as B. quintana by 16S rRNA sequencing; whose plasma yielded >10 molecules/μL of B. quintana cell-free DNA detected with next-generation sequencing (23); or whose serum demonstrated B. quintana or B. henselae IgM or IgG titer results of >1:128. All serologic testing was conducted at the same commercial clinical laboratory.
We reviewed the electronic medical records of patients identified by the laboratories, accessed by using NYC regional health information organizations for clinical and social history details, which included documentation of housing status. The NYC Department of Homeless Services registration database was queried for evidence that cases received services in the NYC shelter system. The NYC Department of Health and Mental Hygiene’s Institutional Review Board determined that the activities described in this article meet the definition of public health surveillance as set forth under 45 CFR§46.102(1)(2).
We identified 5 cases of culture-negative, left-sided B. quintana endocarditis. The 5 patients had experienced unsheltered homelessness within 1 year of admission. None were registered in the NYC shelter system. The case descriptions illustrate similarities in clinical manifestations and highlight the concern of delayed diagnosis.
Case 1 Description
In summer 2022, a middle-aged man with schizophrenia and alcohol use disorder was found unresponsive at an assisted living facility where he had lived for ≈1 year for the management of malnutrition and chronic anemia. According to his family, the patient had previously been living unsheltered in NYC and had been treated for body lice. In the emergency department, the patient was unresponsive but had unremarkable vital signs (Table). Laboratory testing revealed severe anemia and renal failure. Computed tomography (CT) scans of the brain revealed a large, acute, right inferior cerebellar intraparenchymal hemorrhage with surrounding edema. Cerebral angiography revealed a 3-mm right posterior inferior cerebellar artery aneurysm that was treated emergently with endovascular coiling.
Transthoracic echocardiogram (TTE) revealed a 2.2 cm vegetation on the noncoronary aortic valve (AV) leaflet and a 2.0 cm vegetation on the anterior mitral valve leaflet, which is associated with severe regurgitation of both valves with preserved left ventricular function and suggestive of bacterial endocarditis. Healthcare providers collected 1 set of blood cultures before antimicrobial administration and 4 sets the first week of hospitalization. Bacterial cultures were held for 5 days, and fungal cultures were held for 30 days. Cultures remained negative. Providers treated the patient empirically for bacterial endocarditis with ceftriaxone and vancomycin for 12 days, then stopped after blood cultures remained negative. Providers did not initially consider bartonellosis.
The patient received anticoagulants and underwent aortic and mitral bioprosthetic valve replacements 38 days after admission and after neurologic recovery. After reassessment of the case by a new consultant 2 days after surgery, doxycycline was started empirically for possible bartonellosis, and serologic testing was conducted. Results of bacterial culture of the explanted valve tissue was negative; fungal and mycobacterial cultures were not performed. B. henselae and B. quintana IgG titers were >1:1024; IgM titers were negative.
The patient was discharged and then readmitted 3 weeks later with renal failure. Doxycycline monotherapy was continued for 4 weeks and then switched to azithromycin for an additional 2 weeks because of a drug rash while on doxycycline.
After the second hospital discharge, a nonspecific qualitative PCR at a commercial laboratory detected a Bartonella sp. in an explanted AV tissue specimen. The Wadsworth Center conducted Bartonella PCR of whole blood that was collected before doxycycline was started, and the PCR was negative. A Warthin-Starry silver stain of the AV tissue revealed small, black, curved organisms that were consistent with Bartonella spp. (Figure 1).
The patient’s family was contacted after he missed outpatient appointments. They reported that he had left home and was experiencing homelessness. The patient sought treatment at another hospital 2 months after the second discharge. The hospital clinicians diagnosed Clostridioides difficile–associated diarrhea and Enterococcus faecalis bacteremia. No additional Bartonella testing was conducted. The patient was discharged to hospice and died 10 months after his endocarditis diagnosis.
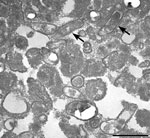
Several months after the patient’s death, formalin-fixed paraffin-embedded explanted AV tissue was submitted to the Centers for Disease Control and Prevention for testing. B. quintana was identified by using a Bartonella-specific hemi-nested PCR targeting the ribC gene (24) followed by sequence analysis of 307 bp and 211 bp PCR products. Electron microscopy revealed B. quintana within aortic tissue (Figure 2).
Case 2 Description
In early spring 2022, a middle-aged man with chronic obstructive pulmonary disease who had been experiencing unsheltered homelessness sought care at an emergency department with dyspnea on exertion that progressed to shortness of breath at rest over the previous 2 weeks. He had a history of bipolar disorder, antisocial personality disorder, and alcohol use disorder. Body lice were discovered and treated after admission.
Physical examination revealed bilateral lower extremity and scrotal edema and diffuse pulmonary crackles. The patient was tachycardic (Table). Laboratory testing revealed hematuria, mild neutrophilic leukocytosis, acute kidney injury, and metabolic acidosis. A chest radiograph showed pulmonary congestion and bilateral pleural effusions. TTE revealed severe aortic regurgitation (AR), a 1.7 cm AV vegetation, a small vegetation on the noncoronary aortic leaflet, elevated end diastolic right ventricular pressure, and preserved left ventricular function.
Healthcare providers obtained blood cultures after a single dose of ceftriaxone and before daptomycin was initiated; the cultures remained negative after 5 days. Clinicians became concerned for possible bartonellosis because of the patient’s recent homelessness and negative blood cultures. Clinicians changed the patient’s antimicrobials to doxycycline and rifampin after serologic testing revealed B. henselae titers of IgG >1:2,560 and B. quintana titers of IgG >1:1,280; IgM titers were negative.
The patient’s renal function continued to decline, likely secondary to glomerulonephritis, and clinicians initiated intermittent dialysis. The patient subsequently refused treatment and laboratory testing but was legally deemed unable to make medical decisions; a court ordered dialysis and cardiothoracic surgery. He underwent bioprosthetic AV replacement and a left atrial appendage ligation on day 29 of hospitalization. The postoperative course was complicated by deterioration of cardiac function and renal failure.
The Wadsworth Center conducted molecular testing of the explanted AV and identified B. quintana by using real-time PCR targeting of the gltA gene and confirmed the B. quintana infection by 16S full gene DNA sequencing of the PCR product. During the hospitalization, the patient completed 10-week courses of doxycycline and rifampin; 6 additional weeks of doxycycline were recommended after hospital discharge. The patient was discharged to a shelter 79 days after admission with hemodialysis scheduled 3 times/week, but he left the facility and did not attend outpatient clinic appointments. He returned to the emergency department 3 months later with volume overload and possible worsening of heart failure (HF), although renal function had recovered. He left against medical advice and was lost to follow-up.
Case 3 Description
An elderly woman with unknown medical history was found disoriented and lying on a subway platform during the winter of 2020. Bed bugs were noted by emergency responders. She was afebrile, bradycardic, and hypotensive, and it was noted she had hyperpigmented lower extremities with 3+ pitting edema (Table). At the hospital, laboratory values revealed moderate leukocytosis, profound anemia, thrombocytopenia, acute kidney injury, elevated pro-B-type natriuretic peptide (pro-BNP), lactic acidosis, and coagulopathy. TTE showed severe AR with large vegetations on the AV noncoronary and right coronary cusps, moderate pulmonary hypertension, moderate tricuspid and severe pulmonic regurgitation, and diastolic dysfunction. Brain magnetic resonance imaging revealed possible embolic-type infarcts in bilateral cerebral and left cerebellar hemispheres.
Healthcare providers collected blood cultures and then administered ceftriaxone and vancomycin empirically for bacterial endocarditis. Providers started the patient on doxycycline and rifampin 3 days later because the negative blood cultures, homelessness, and the ectoparasitic infestation were suggestive for bartonellosis.
The patient underwent urgent tissue AV replacement and tricuspid valve repair. Warthin-Starry stain was negative. Bacterial cultures held 5 days and fungal cultures held 28 days of the explanted valve were negative. Blood cultures were held for 14 days and remained negative.
The titer for B. quintana IgG was <1:128 and the titer for B. henselae IgG was >1:1,024; IgM titers were negative. Clinicians changed the patient’s antimicrobial drugs to doxycycline and rifampin because of the clinical suspicion of bartonellosis after consideration of the elevated Bartonella spp. titers, negative blood cultures, homelessness, and ectoparasitic infestation. The Wadsworth Center conducted molecular testing of the explanted AV and identified B. quintana. The patient died from multiorgan failure 3 weeks after surgery.
Case 4 Description
A middle-aged man with a history of asthma, bipolar disorder, alcohol use disorder, hepatitis C infection, pancytopenia, and experiencing unsheltered homelessness sought care at an emergency department in the winter of 2022 with dyspnea and bilateral lower extremity edema over the previous 2 months. He was hospitalized for fluid overload with suspected acute HF. At admission, the patient’s vital signs were unremarkable, and no signs or symptoms of systemic infection were noted (Table). Laboratory tests revealed anemia, hypoalbuminemia, and elevated pro-BNP. Chest radiograph showed pulmonary congestion and bilateral pleural effusions.
TTE showed AR, mitral regurgitation, tricuspid regurgitation, mild pulmonary hypertension, and suspected mobile vegetations on the AV. Healthcare providers collected blood cultures before initiating treatment with intravenous vancomycin and ceftriaxone for suspected bacterial endocarditis. Initial and repeated blood cultures remained negative after 21 days. Because of the patient’s recent unsheltered homelessness and reported exposure to cats, dogs, and lice, Bartonella endocarditis was considered. Clinicians changed the antimicrobial treatment to doxycycline and rifampin. Serologic testing detected elevated IgG titers to B. henselae of 1:2,560 and B. quintana of 1:640. B. henselae IgM titers were negative.
The patient underwent tissue AV replacement 10 days after admission. Bacterial, fungal, and mycobacterial cultures of the AV tissue were negative. Healthcare providers identified B. quintana from the explanted AV by using Sanger sequencing of amplicons obtained from PCR assays targeting 16S rRNA and ribC genes. The patient was discharged from the hospital and recommended to complete a 12-week course of doxycycline and 6-week course of rifampin. However, he continued to experience unsheltered homelessness and reported that he was unable to complete the recommended regimen because his medications were stolen.
Case 5 Description
An ≈60-year-old man experiencing unsheltered homelessness was admitted to an emergency department in summer 2023 after being found collapsed on a sidewalk. Physical examination revealed left-sided facial droop and weakness. He was afebrile, and vital signs were unremarkable. Laboratory testing showed severe anemia and normal renal function (Table). CT scans of the patient’s head revealed an acute right middle cerebral artery infarct with suspected thrombus. CT angiography showed occlusion of the right M1 segment, which was treated with thrombectomy.
Transesophageal echocardiogram revealed mobile vegetations on each AV leaflet, anterior and posterior mitral valve leaflets, and the tricuspid valve; mild AR; and mild to moderate mitral regurgitation. Digital subtraction angiography revealed a 3 mm distal middle cerebral artery mycotic aneurysm.
Healthcare providers collected blood cultures and initiated vancomycin and ceftriaxone for suspected bacterial endocarditis. Blood cultures were held for 5 days and remained negative.
Clinicians considered the patient at risk for B. quintana endocarditis because he experienced recent homelessness. They added doxycycline and rifampin after B. hensalae and B. quintana IgG titers were both 1:2,560; IgM titers were negative. Clinicians discontinued vancomycin and ceftriaxone when cell-free B. quintana DNA was detected in the patient’s plasma by using next-generation sequencing.
The patient completed 6 weeks of rifampin and 7 weeks of doxycycline during hospitalization. He did not undergo valve replacement because of stable vegetations and high risk for aneurysm hemorrhage if anticoagulated. He was discharged wheelchair-bound to a shelter. After falling from his wheelchair the next day, he was hospitalized and completed his doxycycline course during an extended rehabilitation hospitalization.
We describe 5 cases of left-sided B. quintana endocarditis that occurred during 2020–2023 in persons experiencing unsheltered homelessness in NYC. Most patients had serious complications, including 4 with HF, 3 with renal failure, and 2 with embolic strokes. Renal replacement therapy during hospitalization was required for 2 cases, and 2 patients died.
Echocardiographic findings of AV vegetations prompted evaluations for infective endocarditis. In all cases, negative blood cultures triggered testing for Bartonella spp. because clinicians recognized that B. quintana infection was a possibility because of the patients’ history of experiencing homelessness. Body lice and homelessness have been linked to B. quintana infection (3,9–12,17,19). In this article, body louse exposure was documented in 3 of 5 endocarditis cases; a fourth patient had what appeared to be bed bugs, which might have been misidentified body lice.
Because of nonspecific clinical manifestations, B. quintana endocarditis requires a high index of clinical suspicion to diagnose. Even when clinicians consider bartonellosis, diagnosis can be elusive. The bacterium stains poorly with Gram stain and is visualized better with Giemsa or Warthin-Starry silver stain, although histopathological diagnosis is insensitive and nonspecific (5). Diagnosis is commonly made by using serology but can be challenging. Cross-reactivity between different Bartonella spp. can affect interpretation (1,16,25–27). In the cases reported in this article, titers for B. henselae IgG were equal to or higher than for B. quintana IgG, and in 1 endocarditis case, B. quintana IgG was negative. B. henselae and B. quintana IgM titers were also negative in all cases, which is expected with prolonged chronic infections.
A standard approach to B. quintana diagnosis is lacking. As fastidious organisms, Bartonella spp. are difficult to grow by using routine blood culture methods and require up to 3–4 weeks of incubation. Bartonella spp. endocarditis blood culture sensitivity can be <20% (1,28). Periodic subculturing of blood culture broth in shell vials or to solid media with different base formulas has been used, but most clinical laboratories are unable to use these specialized techniques (1,4,29). None of the B. quintana endocarditis cases in this article were detected by blood culture and in 4/5 cases cultures were only held for 5 days.
Molecular assays, such as PCR amplification of specific gene targets (e.g., the Bartonella citrate synthase gene, gltA, ribC) and 16S rRNA sequencing of PCR products, are the most reliable methods to detect Bartonella infection and can be used for blood or fresh tissue specimens (4,27,30,31). In addition, molecular assays can be performed on formalin-fixed paraffin-embedded tissues for retrospective analysis. The diagnostic sensitivity of PCR to detect B. quintana from cardiac valve tissue in 1 reference center was 81%, compared with 28% for blood to shell vial culture, 44% for valve tissue to shell vial culture, 33% for blood, and 36% for serum (4,5). However, molecular diagnosis of Bartonella spp. is available in only a limited number of public health and commercial laboratories and tissue is not always available for testing.
In this investigation, molecular testing of excised valve tissue confirmed the detection of B. quintana endocarditis in 4 patients, including in 1 patient whose PCR blood test was negative and another with undetectable B. quintana IgG. Detection of cell-free B. quintana DNA in plasma confirmed the diagnosis in the patient who did not undergo valve replacement.
B. quintana infection results in intraerythrocytic and endothelial propagation with biofilm formation that protects it from the immune response and antimicrobial drugs, which might explain the insidious consequences of prolonged bacteremia, resistance to many antibiotic classes, and propensity for recurrent infection (1). Experimental and clinical evidence suggest that aminoglycosides have bactericidal activity against Bartonella spp. and should be used for >14 days, ideally in combination with a second antimicrobial drug (32). Doxycycline plus rifampin has been recommended as an effective alternative regimen (33). For B. quintana endocarditis, 4–6 weeks of combination therapy is recommended (1,33,34), and valve replacement is often necessary.
A low threshold for diagnostic testing and administration of empiric antimicrobial drugs is necessary when B. quintana infection is suspected. Because infections can be asymptomatic and prolonged, the diagnosis and treatment of bartonellosis can be challenging, the consequences of untreated infection can be severe, untreated infection poses a risk of subsequent transmission, and the cost for each prolonged hospitalization (mean = 45 days in this article) is great. Persons experiencing homelessness who are unable to maintain regular clothing hygiene are at an increased risk for body louse infestation and B. quintana infection. When evaluating patients with vasoproliferative skin lesions, HF, or cardiac valve vegetations, clinicians should routinely ask about housing status and body lice exposure. In this investigation, clinicians started B. quintana–directed antimicrobial drugs for 3 patients within 4 days of their presumptive bacterial endocarditis detection. Those clinicians were in a hospital that frequently treats persons experiencing homelessness.
A history of alcohol use disorder was discovered in 3 of 4 cases with known medical histories, which has been documented previously in patients with B. quintana endocarditis (5,14,16,20). Those patients also had mental health conditions. Those related conditions may have contributed to delayed detection and poor outcomes.
The burden of B. quintana infection in persons experiencing homelessness is unknown. However, we infer from the published literature that this burden might be considerable. In 1999, 25% of clinic patients experiencing homelessness in Seattle, Washington, USA, had elevated antibody titers to B. quintana, compared with 1% of blood donor controls (16). During 2016–2021, medical centers in Denver, Colorado, USA, reported 12 (85.7%) of 14 patients with B. quintana infections were experiencing homelessness (20). Over 1-year in Marseille, France, B. quintana bacteremia was detected in 10 (14%) of 71 of all patients experiencing homelessness (3). With an estimated 653,100 persons experiencing homelessness in the United States (35), the potential burden is considerable.
Bartonellosis is not a nationally notifiable disease and is not reportable to the NYC Department of Health and Mental Hygiene. Persons at risk for B. quintana infection are also frequently outside the reach of healthcare. Moreover, even if a patient is suspected to have bartonellosis, diagnostic testing is often inconclusive or negative. Therefore, it is likely those cases represent a fraction of all B. quintana infections in unsheltered NYC residents during that period.
In conclusion, clinicians who provide clinical services to persons experiencing homelessness should consider B. quintana infection when patients have symptoms that may be consistent with bartonellosis, particularly in the setting of prior or current body louse infestation. Available diagnostic testing includes blood and tissue cultures, serologic testing, and molecular assays, which includes cell-free DNA testing; however, negative results do not entirely rule out B. quintana infection, and empiric antimicrobial drug treatment should be considered if clinical suspicion for B. quintana infection is high.
Dr. Keller is an infectious diseases clinician and the associate hospital epidemiologist at Westchester Medical Center in Valhalla, New York. Her research interests include hospital acquired infections, public health, and data analytics.
Acknowledgment
We thank the following people for their contributions to this investigation: Sally Slavinski, Camille Bergeron-Parent, Dan Ting Chen, Sarah Park, Leah Seifu, Amy Beeson, and Roosecelis B. Martines.
References
- Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. 2017;30:709–46. DOIPubMedGoogle Scholar
- Brouqui P, Raoult D. Arthropod-borne diseases in homeless. Ann N Y Acad Sci. 2006;1078:223–35. DOIPubMedGoogle Scholar
- Brouqui P, Lascola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–9. DOIPubMedGoogle Scholar
- La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37:1899–905. DOIPubMedGoogle Scholar
- Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol. 2015;53:824–9. DOIPubMedGoogle Scholar
- Raoult D, Fournier PE, Vandenesch F, Mainardi JL, Eykyn SJ, Nash J, et al. Outcome and treatment of Bartonella endocarditis. Arch Intern Med. 2003;163:226–30. DOIPubMedGoogle Scholar
- Deng YP, Fu YT, Yao C, Shao R, Zhang XL, Duan DY, et al. Emerging bacterial infectious diseases/pathogens vectored by human lice. Travel Med Infect Dis. 2023;55:
102630 . DOIPubMedGoogle Scholar - Anstead GM. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1. Lancet Infect Dis. 2016;16:e164–72. DOIPubMedGoogle Scholar
- Bonilla DL, Kabeya H, Henn J, Kramer VL, Kosoy MY. Bartonella quintana in body lice and head lice from homeless persons, San Francisco, California, USA. Emerg Infect Dis. 2009;15:912–5. DOIPubMedGoogle Scholar
- Bonilla DL, Cole-Porse C, Kjemtrup A, Osikowicz L, Kosoy M. Risk factors for human lice and bartonellosis among the homeless, San Francisco, California, USA. Emerg Infect Dis. 2014;20:1645–51. DOIPubMedGoogle Scholar
- Brouqui P, Stein A, Dupont HT, Gallian P, Badiaga S, Rolain JM, et al. Ectoparasitism and vector-borne diseases in 930 homeless people from Marseilles. Medicine (Baltimore). 2005;84:61–8. DOIPubMedGoogle Scholar
- Brouqui P, Houpikian P, Dupont HT, Toubiana P, Obadia Y, Lafay V, et al. Survey of the seroprevalence of Bartonella quintana in homeless people. Clin Infect Dis. 1996;23:756–9. DOIPubMedGoogle Scholar
- Raoult D, Fournier PE, Drancourt M, Marrie TJ, Etienne J, Cosserat J, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–52. DOIPubMedGoogle Scholar
- Spach DH, Kanter AS, Dougherty MJ, Larson AM, Coyle MB, Brenner DJ, et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–8. DOIPubMedGoogle Scholar
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131–5. DOIPubMedGoogle Scholar
- Jackson LA, Spach DH, Kippen DA, Sugg NK, Regnery RL, Sayers MH, et al. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J Infect Dis. 1996;173:1023–6. DOIPubMedGoogle Scholar
- Faccini-Martínez AA, Kmetiuk LB, Blanton LS, Felipetto LG, Gravinatti ML, Timenetsky J, et al. Bartonella spp. and typhus group rickettsiae among persons experiencing homelessness, São Paulo, Brazil. Emerg Infect Dis. 2023;29:418–21. DOIPubMedGoogle Scholar
- Leibler JH, Zakhour CM, Gadhoke P, Gaeta JM. Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990–2014. Vector Borne Zoonotic Dis. 2016;16:435–44. DOIPubMedGoogle Scholar
- Marshall KE, Martinez HE, Woodall T, Guerrero A, Mechtenberg J, Herlihy R, et al. Body lice among people experiencing homelessness and access to hygiene services during the COVID-19 pandemic—preventing trench fever in Denver, Colorado, 2020. Am J Trop Med Hyg. 2022;107:427–32. DOIPubMedGoogle Scholar
- Shepard Z, Vargas Barahona L, Montalbano G, Rowan SE, Franco-Paredes C, Madinger N. Bartonella quintana infection among people experiencing homelessness in the Denver metropolitan area. J Infect Dis. 2022;226(Suppl 3):S315–21. DOIPubMedGoogle Scholar
- Beeson AM, Rich SN, Russo ME, Bhatnagar J, Kumar RN, Ritter JM, et al. Bartonella quintana infection in kidney transplant recipients from donor experiencing homelessness, United States, 2022. Emerg Infect Dis. 2024;30:XXX–XX.
- Rich SN, Beeson A, Seifu L, Mitchell K, Wroblewski D, Juretschko S, et al. Severe Bartonella quintana infections among persons experiencing unsheltered homelessness—New York City, January 2020–December 2022. MMWR Morb Mortal Wkly Rep. 2023;72:1147–8. DOIPubMedGoogle Scholar
- Park SY, Chang EJ, Ledeboer N, Messacar K, Lindner MS, Venkatasubrahmanyam S, et al. Plasma microbial cell-free DNA sequencing from over 15,000 patients identified a broad spectrum of pathogens. J Clin Microbiol. 2023;61:
e0185522 . DOIPubMedGoogle Scholar - Zeaiter Z, Fournier P-E, Greub G, Raoult D. Diagnosis of Bartonella endocarditis by a real-time nested PCR assay using serum. J Clin Microbiol. 2003;41:919–25. DOIPubMedGoogle Scholar
- Maurin M, Eb F, Etienne J, Raoult D. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J Clin Microbiol. 1997;35:2283–7. DOIPubMedGoogle Scholar
- Fournier P-E, Mainardi J-L, Raoult D. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol. 2002;9:795–801.PubMedGoogle Scholar
- Rahimian J, Raoult D, Tang YW, Hanna BA. Bartonella quintana endocarditis with positive serology for Coxiella burnetii. J Infect. 2006;53:e151–3. DOIPubMedGoogle Scholar
- Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84:162–73. DOIPubMedGoogle Scholar
- Gouriet F, Fenollar F, Patrice J-Y, Drancourt M, Raoult D. Use of shell-vial cell culture assay for isolation of bacteria from clinical specimens: 13 years of experience. J Clin Microbiol. 2005;43:4993–5002. DOIPubMedGoogle Scholar
- McCormick DW, Rassoulian-Barrett SL, Hoogestraat DR, Salipante SJ, SenGupta D, Dietrich EA, et al. Bartonella spp. infections identified by molecular methods, United States. Emerg Infect Dis. 2023;29:467–76. DOIPubMedGoogle Scholar
- Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, et al. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg. 2010;82:1140–5. DOIPubMedGoogle Scholar
- Foucault C, Raoult D, Brouqui P. Randomized open trial of gentamicin and doxycycline for eradication of Bartonella quintana from blood in patients with chronic bacteremia. Antimicrob Agents Chemother. 2003;47:2204–7. DOIPubMedGoogle Scholar
- Rolain J-M, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921–33. DOIPubMedGoogle Scholar
- Rolain J-M, Maurin M, Raoult D. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J Antimicrob Chemother. 2000;46:811–4. DOIPubMedGoogle Scholar
- US Department of Housing and Urban Development. The 2023 Annual Homelessness Assessment Report (AHAR) to Congress. Part 1: point-in-time estimates of homelessness. 2023. [cited 2024 Sep 25] https://www.huduser.gov/portal/sites/default/files/pdf/2023-AHAR-Part-1.pdf
Figures
Table
Cite This ArticleOriginal Publication Date: November 20, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 12—December 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Joel Ackelsberg, New York City Department of Health and Mental Hygiene, 42-09 28th St, CN-22, Long Island City, NY 11101, USA
Top